Chemistry
Most local anesthetic drugs are esters or amides of simple benzene derivatives. Subgroups within the local anesthetics are based on this chemical characteristic and on duration of action. The commonly used local anesthetics are weak bases with at least 1 ionizable amine function that can become charged through the gain of a proton (H+). As discussed in Chapter 1, the degree of ionization is a function of the pKa of the drug and the pH of the medium. Because the pH of tissue may differ from the physiologic 7.4 (eg, it may be as low as 6.4 in infected tissue), the degree of ionization of the drug will vary. Because the pKa of most local anesthetics is between 8.0 and 9.0 (benzocaine is an exception), variations in pH associated with infection can have significant effects on the proportion of ionized to nonionized drug. The question of the active form of the drug (ionized versus nonionized) is discussed later.
Pharmacokinetics
Many shorter-acting local anesthetics are readily absorbed into the blood from the injection site after administration. The duration of local action is therefore limited unless blood flow to the area is reduced. This can be accomplished by administration of a vasoconstrictor (usually an  -agonist sympathomimetic) with the local anesthetic agent. Cocaine is an important exception because it has intrinsic sympathomimetic action due to its inhibition of norepinephrine reuptake into nerve terminals. The longer-acting agents (eg, bupivicaine, ropivicaine, tetracain) are also less dependent on the coadministration of vasoconstrictors. Surface activity (ability to reach superficial nerves when applied to the surface of mucous membranes) is a property of certain local anesthetics, especially cocaine and benzocaine (both only available as topical forms), lidocaine, and tetracaine.
-agonist sympathomimetic) with the local anesthetic agent. Cocaine is an important exception because it has intrinsic sympathomimetic action due to its inhibition of norepinephrine reuptake into nerve terminals. The longer-acting agents (eg, bupivicaine, ropivicaine, tetracain) are also less dependent on the coadministration of vasoconstrictors. Surface activity (ability to reach superficial nerves when applied to the surface of mucous membranes) is a property of certain local anesthetics, especially cocaine and benzocaine (both only available as topical forms), lidocaine, and tetracaine.
Metabolism of ester local anesthetics is carried out by plasma cholinesterases (pseudocholinesterases) and is very rapid for procaine (half-life, 1-2 min), slower for cocaine, and very slow for tetracaine). The amides are metabolized in the liver, in part by cytochrome P450 isozymes. The half-lives of lidocaine and prilocaine are approximately 1.5 h. Bupivacaine and ropivacaine are the longest-acting amide local anesthetics with half-lives of 3.5 and 4.2 h, respectively. Liver dysfunction may increase the elimination half-life of amide local anesthetics (and increase the risk of toxicity).
Acidification of the urine promotes ionization of local anesthetics; the charged forms of such drugs are more rapidly excreted than nonionized forms.
Mechanism of Action
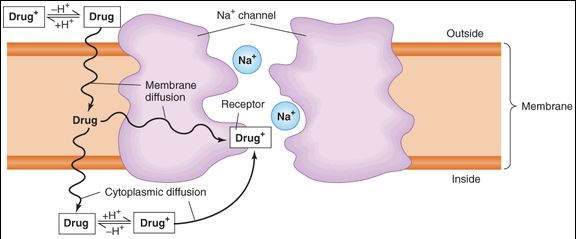
Local anesthetics block voltage-dependent sodium channels and reduce the influx of sodium ions, thereby preventing depolarization of the membrane and blocking conduction of the action potential. Local anesthetics gain access to their receptors from the cytoplasm or the membrane (Figure 26-1). Because the drug molecule must cross the lipid membrane to reach the cytoplasm, the more lipid-soluble (nonionized, uncharged) form reaches effective intracellular concentrations more rapidly than does the ionized form. On the other hand, once inside the axon, the ionized (charged) form of the drug is the more effective blocking entity. Thus, both the nonionized and the ionized forms of the drug play important roles—the first in reaching the receptor site and the second in causing the effect. The affinity of the receptor site within the sodium channel for the local anesthetic is a function of the state of the channel, whether it is resting, open, or inactivated, and therefore follows the same rules of use dependence and voltage dependence that were described for the sodium channel-blocking antiarrhythmic drugs (see Chapter 14). In particular, if other factors are equal, rapidly firing fibers are usually blocked before slowly firing fibers. High concentrations of extracellular K+ may enhance local anesthetic activity, whereas elevated extracellular Ca2+ may antagonize it.
FIGURE 26-1
Schematic diagram of the sodium channel in an excitable membrane (eg, an axon) and the pathways by which a local anesthetic molecule (Drug
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree