Local Anesthetics
Local anesthetics bind reversibly to a specific receptor site within the pore of the Na+ channels in nerves and block ion movement through this pore. When applied locally to nerve tissue in appropriate concentrations, local anesthetics can act on any part of the nervous system and on every type of nerve fiber, reversibly blocking the action potentials responsible for nerve conduction.
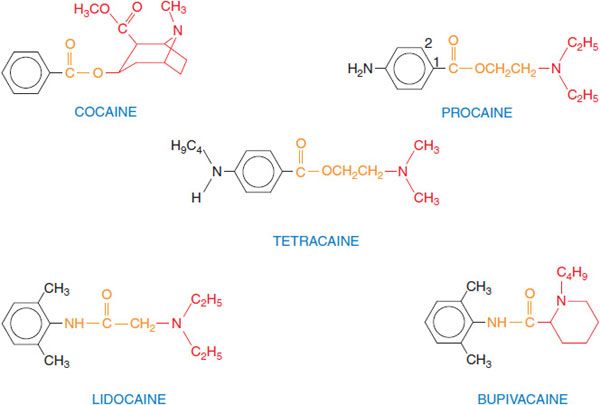
CHEMISTRY AND STRUCTURE-ACTIVITY RELATIONSHIP. The most widely used agents today are procaine, lidocaine, bupivacaine, and tetracaine (Figure 20–1). These agents were synthesized as substitutes for cocaine, preserving the local anesthetic effect of cocaine but avoiding its toxicity and addictive properties. The typical local anesthetics contain hydrophilic and hydrophobic moieties that are separated by an intermediate ester or amide linkage. The hydrophilic group usually is a tertiary amine but also may be a secondary amine; the hydrophobic moiety must be aromatic. The nature of the linking group determines some of the pharmacological properties of these agents. For example, local anesthetics with an ester link are hydrolyzed readily by plasma esterases. Hydrophobicity increases both the potency and the duration of action of the local anesthetics; association of the drug at hydrophobic sites enhances the partitioning of the drug to its sites of action and decreases the rate of metabolism by plasma esterases and hepatic enzymes. In addition, the receptor site for these drugs on Na+ channels is thought to be hydrophobic, so that receptor affinity for anesthetic agents is greater for more hydrophobic drugs. Hydrophobicity also increases toxicity, so that the therapeutic index is decreased for more hydrophobic drugs.
Figure 20–1 Structural formulas of selected local anesthetics. Most local anesthetics consist of a hydrophobic (aromatic) moiety (black), a linker region (orange), and a substituted amine (hydrophilic region, in red). Procaine is a prototypic ester-type local anesthetic; esters generally are well hydrolyzed by plasma esterases, contributing to the relatively short duration of action of drugs in this group. Lidocaine is a prototypic amide-type local anesthetic; these structures generally are more resistant to clearance and have longer durations of action. Figure 20–1 in 12th edition of the parent text shows additional variations on the basic structure.
Molecular size influences the rate of dissociation of local anesthetics from their receptor sites. Smaller drug molecules can escape from the receptor site more rapidly. This characteristic is important in rapidly firing cells, in which local anesthetics bind during action potentials and dissociate during the period of membrane repolarization. Rapid binding of local anesthetics during action potentials causes the frequency- and voltage-dependence of their action.
MECHANISM OF ACTION. Local anesthetics act at the cell membrane to prevent the generation and the conduction of nerve impulses. The major mechanism of action of local anesthetics involves their interaction with 1 or more specific binding sites within the Na+ channel (see Figure 14–1A).
Local anesthetics block conduction by decreasing or preventing the large transient increase in the permeability of excitable membranes to Na+ that normally is produced by membrane depolarization. This action of local anesthetics is due to their direct interaction with voltage-gated Na+ channels. As the anesthetic action progressively develops in a nerve, the threshold for electrical excitability gradually increases, the rate of rise of the action potential declines, impulse conduction slows, and nerve conduction fails. Local anesthetics can block K+ channels but this interaction requires higher concentrations of drug; thus, blockade of conduction is not accompanied by any large change in resting membrane potential.
FREQUENCY- AND VOLTAGE-DEPENDENCE OF LOCAL ANESTHETIC ACTION. A higher frequency of stimulation and more positive membrane potential cause a greater degree of anesthetic block. These frequency- and voltage-dependent effects of local anesthetics occur because the local anesthetic molecule in its charged form, gains access to its binding site within the pore only when the Na+ channel is in an open state, and because the local anesthetic binds more tightly to and stabilizes the inactivated state of the Na+ channel. The frequency dependence of local anesthetic action depends critically on the rate of dissociation from the receptor site in the pore of the Na+ channel. A high frequency of stimulation is required for rapidly dissociating drugs so that drug binding during the action potential exceeds drug dissociation between action potentials.
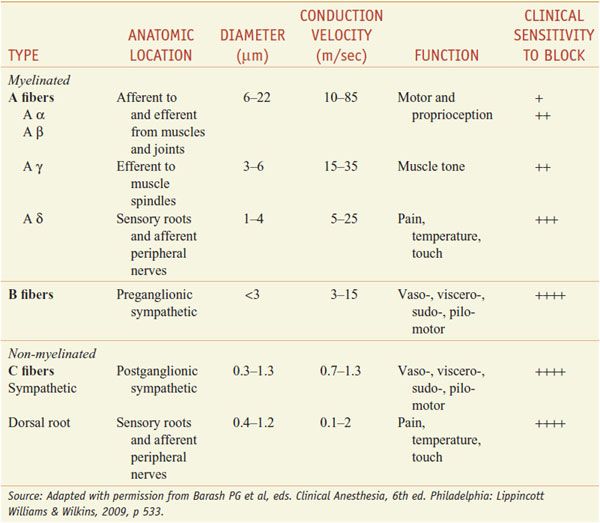
DIFFERENTIAL SENSITIVITY OF NERVE FIBERS TO LOCAL ANESTHETICS. For most patients treatment with local anesthetics causes the sensation of pain to disappear first, followed by loss of the sensations of temperature, touch, deep pressure, and finally motor function (Table 20–1).
Table 20–1
Susceptibility of Nerve Fibers to Local Anesthetics
In general, autonomic fibers, small unmyelinated C fibers (mediating pain sensations), and small myelinated Aδ fibers (mediating pain and temperature sensations) are blocked before the larger myelinated Aγ, Aβ, and Aα fibers (mediating postural, touch, pressure, and motor information) The precise mechanisms responsible for this apparent specificity of local anesthetic action on pain fibers are not known. The differential rate of block exhibited by fibers mediating different sensations is of considerable practical importance in the use of local anesthetics.
EFFECT OF pH. Local anesthetics tend to be only slightly soluble as unprotonated amines. Therefore, they generally are marketed as water-soluble salts, usually hydrochlorides. Inasmuch as the local anesthetics are weak bases (typical pKa values range from 8-9), their hydrochloride salts are mildly acidic. This property increases the stability of the local anesthetic esters and the catecholamines added as vasoconstrictors. Under usual conditions of administration, the pH of the local anesthetic solution rapidly equilibrates to that of the extracellular fluids.
PROLONGATION OF ACTION BY VASOCONSTRICTORS. The duration of action of a local anesthetic is proportional to the time of contact with nerve. Consequently, maneuvers that keep the drug at the nerve prolong the period of anesthesia. In clinical practice, a vasoconstrictor, usually epinephrine, is often added to local anesthetics. The vasoconstrictor, by decreasing the rate of absorption, localizes the anesthetic at the desired site, and reduces systemic toxicity by allowing metabolism to keep pace with the rate at which it is absorbed into the circulation. It should be noted, however, that epinephrine also dilates skeletal muscle vascular beds through actions at β2 adrenergic receptors, and therefore has the potential to increase systemic toxicity of anesthetic deposited in muscle tissue.
UNDESIRED EFFECTS OF LOCAL ANESTHETICS. Local anesthetics interfere with the function of all organs in which conduction or transmission of impulses occurs. Thus, they have important effects on the CNS, the autonomic ganglia, the neuromuscular junction, and all forms of muscle.
The danger of such adverse reactions is proportional to the concentration of local anesthetic achieved in the circulation. In general, in local anesthetics with chiral centers, the S-enantiomer is less toxic than the R-enantiomer.
CNS. Following absorption, local anesthetics may cause CNS stimulation, producing restlessness and tremor that may progress to clonic convulsions. Central stimulation is followed by depression; death usually is caused by respiratory failure. Benzodiazepines or rapidly acting barbiturates administered intravenously are the drugs of choice for both the prevention and arrest of convulsions (see Chapter 17). Lidocaine may produce dysphoria or euphoria and muscle twitching. Moreover, both lidocaine and procaine may produce a loss of consciousness that is preceded only by symptoms of sedation.
Cardiovascular System. Following systemic absorption, local anesthetics act on the cardiovascular system, primarily on the myocardium, where decreases in electrical excitability, conduction rate, and force of contraction occur. In addition, most local anesthetics cause arteriolar dilation. Untoward cardiovascular effects usually are seen only after high systemic concentrations are attained and effects on the CNS are produced; on rare occasions, lower doses of some local anesthetics will cause cardiovascular collapse and death. Ventricular tachycardia and fibrillation are relatively uncommon consequences of local anesthetics other than bupivacaine. Untoward cardiovascular effects of local anesthetic agents may result from their inadvertent intravascular administration, especially if epinephrine also is present.
Smooth Muscle. Local anesthetics depress contractions in the bowel. They also relax vascular and bronchial smooth muscle, although low concentrations initially may produce contraction. Spinal and epidural anesthesia and instillation of local anesthetics into the peritoneal cavity cause sympathetic nervous system paralysis, which can result in increased tone of GI musculature. Local anesthetics seldom depress uterine contractions during intrapartum regional anesthesia.
Neuromuscular Junction and Ganglionic Synapse. Local anesthetics also affect transmission at the neuromuscular junction. Procaine, e.g., can block the response of skeletal muscle to ACh at concentrations at which the muscle responds normally to direct electrical stimulation. Similar effects occur at autonomic ganglia. These effects are due to block of nicotinic ACh receptors by high concentrations of the local anesthetic.
HYPERSENSITIVITY TO LOCAL ANESTHETICS. Rare individuals are hypersensitive to local anesthetics. The reaction may manifest itself as an allergic dermatitis or a typical asthmatic attack. Hypersensitivity seems to occur more frequently with local anesthetics of the ester type and frequently extends to chemically related compounds. Local anesthetic preparations containing a vasoconstrictor also may elicit allergic responses due to the sulfite added as an antioxidant for the catecholamine/vasoconstrictor.
METABOLISM OF LOCAL ANESTHETICS. The metabolic fate of local anesthetics is of great practical importance, because their toxicity depends largely on the balance between their rates of absorption and elimination. The rate of absorption of many anesthetics can be reduced considerably by the incorporation of a vasoconstrictor agent in the anesthetic solution. However, the rate of degradation of local anesthetics varies greatly, and this is a major factor in determining the safety of a particular agent. Because toxicity is related to the concentration of free drug, binding of the anesthetic to proteins in the serum and to tissues reduces the concentration of free drug in the systemic circulation, and consequently reduces toxicity.
Some of the common local anesthetics (e.g., tetracaine) are esters; they are inactivated primarily by a plasma esterase. The liver also participates in hydrolysis of local anesthetics. Because spinal fluid contains little or no esterase, anesthesia produced by the intrathecal injection of an anesthetic agent will persist until the local anesthetic agent has been absorbed into the circulation. The amide-linked local anesthetics are, in general, degraded by the hepatic CYPs the initial reactions involving N-dealkylation and subsequent hydrolysis. With prilocaine, the initial step is hydrolytic, forming o-toluidine metabolites that can cause methemoglobinemia. The extensive use of amide-linked local anesthetics in patients with severe hepatic disease requires caution. The amide-linked local anesthetics are extensively (55-95%) bound to plasma proteins, particularly α1-acid glycoprotein. Many factors increase (e.g., cancer, surgery, trauma, myocardial infarction, smoking, and uremia) or decrease (e.g., oral contraceptives) the level of this glycoprotein, thereby changing the amount of anesthetic delivered to the liver for metabolism and thus influencing systemic toxicity. Age-related changes in protein binding of local anesthetics also occur. The neonate is relatively deficient in plasma proteins that bind local anesthetics and thereby is more susceptible to toxicity. Uptake by the lung also may play an important role in the distribution of amide-linked local anesthetics in the body. Reduced cardiac output slows delivery of the amide compounds to the liver, reducing their metabolism and prolonging their plasma half-lives.
COCAINE
Cocaine, an ester of benzoic acid and methylecgonine, occurs in abundance in the leaves of the coca shrub.
PHARMACOLOGICAL ACTIONS AND PREPARATIONS. The clinically desired actions of cocaine are the blockade of nerve impulses, as a consequence of its local anesthetic properties, and local vasoconstriction, secondary to inhibition of local NE reuptake. Cocaine’s high toxicity is due to reduced catecholamine uptake in both the central and peripheral nervous systems. Its euphoric properties are due primarily to inhibition of catecholamine uptake, particularly DA, in the CNS. Cocaine is used primarily for topical anesthesia of the upper respiratory tract, where its combination of both vasoconstrictor and local anesthetic properties provide anesthesia and shrinking of the mucosa. Cocaine hydrochloride is provided as a 1%, 4%, or 10% solution for topical application. Because of its abuse potential, cocaine is listed as a schedule II controlled substance by the U.S. Drug Enforcement Agency.
LIDOCAINE
Lidocaine (XYLOCAINE, others), an aminoethylamide, is the prototypical amide local anesthetic.
PHARMACOLOGICAL ACTIONS; PREPARATIONS. Lidocaine produces faster, more intense, longer-lasting, and more extensive anesthesia than does an equal concentration of procaine. Lidocaine is an alternative choice for individuals sensitive to ester-type local anesthetics. Lidocaine is absorbed rapidly after parenteral administration and from the GI and respiratory tracts. Although it is effective when used without any vasoconstrictor, epinephrine decreases the rate of absorption, such that the toxicity is decreased and the duration of action usually is prolonged. In addition to preparations for injection, lidocaine is formulated for topical, ophthalmic, mucosal, and transdermal use.
A lidocaine transdermal patch (LIDODERM) is used for relief of pain associated with postherpetic neuralgia. An oral patch (DENTIPATCH) is available for application to accessible mucous membranes of the mouth prior to superficial dental procedures. The combination of lidocaine (2.5%) and prilocaine (2.5%) in an occlusive dressing (EMLA, others) is used as an anesthetic prior to venipuncture, skin graft harvesting, and infiltration of anesthetics into genitalia. Lidocaine in combination with tetracaine (PLIAGLIS
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree