74
CHAPTER OUTLINE
The liver is commonly affected by regular or heavy use of alcohol or illicit drug use. This may be the result of direct toxicity or metabolic or immunologic damage initiated by drug use or due to extraneous factors such as viral hepatitis or bacterial infections acquired through drug use. This chapter describes the more common liver diseases associated with use of alcohol and other drugs (Table 74-1). The emphasis is on clinical manifestations, diagnosis, and management.
TABLE 74-1 ASSOCIATIONS BETWEEN DRUGS OF ABUSE AND LIVER DISEASE
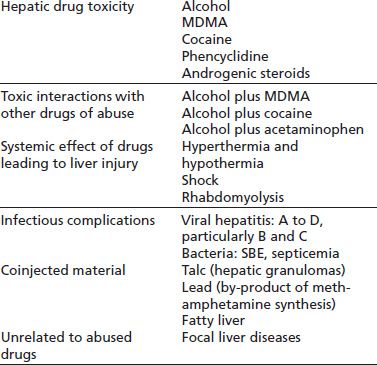
MDMA, 3,4-methylenedioxymethamphetamine. SBE, subacute bacterial endocarditis
ALCOHOL-RELATED LIVER DISEASE
Alcohol-related liver disease (ALD) is the major cause of advanced liver disease in Western countries and a major global cause of morbidity and mortality. The complexity of the pathogenesis of ALD and the apparent ability of some individuals to drink large amounts of alcohol without overt harm continues to challenge our understanding of this disorder. Once alcoholic injury has been initiated, it may progress even if alcohol intake is reduced or ceased particularly in the presence of cofactors discussed below. This demands early detection of this form of liver disease. Treatment of alcohol dependence is one of the few effective strategies to assist the patient recovering from ALDs. Early detection of use disorders and organ damage followed by specific management strategies does improve patient outcomes.
Epidemiology
Cirrhosis of the liver is the 12th most common cause of death in America and the fifth most common cause among middle-aged American men. It accounted for 31,802 deaths in 2010, of which approximately 50% were alcohol related (15,950), with an associated rate of death 5.2/100,000 (1,2). ALD is the second most common indication for liver transplantation in the United States. When it is recognized that many hepatitis C patients requiring transplantation also drink or have drunk heavily, the full impact of alcohol-related liver disease on health care services can be appreciated (3,4).
ALD represents a spectrum of disease ranging from alcohol-related steatosis to cirrhosis, and only a subset of patients consuming harmful levels of alcohol will develop advanced liver disease and cirrhosis. There are a number of recognized risk factors that are associated with progression of ALD; some of these are related to patterns of alcohol consumption, whereas others such as gender, superimposed liver insults, genetic factors, ingestion of hepatotoxins, and nutrition are not.
Risk Factors for Alcohol-Related Liver Disease
Amount of Alcohol Consumed
The population risk of alcohol-related cirrhosis is closely related to the population level of alcohol consumption. This phenomenon has been demonstrated in many countries including the United States and various European and Scandinavian states. A rise or fall in national per capita intake is followed by a variable lag before the benefit or adverse effects are seen clinically (5,6). For example, cirrhosis mortality strikingly reduced during the prohibition era in the United States. The prevalence of alcohol cirrhosis has doubled in the last decade in the United Kingdom in parallel with increased population alcohol use, whereas falling levels of intake in France and Australia have seen a fall in cirrhosis incidence in these countries (7). It is important to stress that these assumptions depend on accurate consumption figures and these can vary depending on sources used and assumptions made. A recent report found rising hospital admissions for ALD despite apparently stable levels of alcohol use (8). Subsequent research found that Australian consumption may have risen in the past decade due to the rising alcohol content of wine not picked up by official data (9).
Reversible fatty liver may be observed after a single heavy drinking episode, but progression to more advanced liver disease usually requires regular daily intake of more than 40 g/d for several (>10) years (10,11). Population studies demonstrate an increasing risk of cirrhosis above 40 g/d for men and 20 g/d for women, but the majority of those with cirrhosis have drunk more than 100 g/d (10,12). The risk of severe liver damage rises to approximately 50% but does not reach 100% even at the highest levels of alcohol consumption.
Gender
Women appear to be at greater risk of ALD for a given level of alcohol consumption, with that risk becoming evident at 20 g/d compared to 40g/d for men (10,11,13–15). Many factors contribute to the difference in apparent susceptibility between men and women, but none fully explains this finding. Differences in body composition, average weight, gastric alcohol dehydrogenase (ADH) activity (accounting, in part, for first-pass, gastric alcohol metabolism), hepatic alcohol metabolic rate, and liver mass per kilogram of body weight between men and women result in a higher relative alcohol dose in women compared to men drinking the same amount. This is reflected in a higher blood alcohol level for a given amount of alcohol (16,17). Data suggesting that women have a higher alcohol metabolism rate than men may reflect both a testosterone-mediated down-regulation of hepatic ADH in men (18) and a higher liver mass per kilogram of body weight in women (19). There is also a gender difference in endotoxin-induced Kupffer cell activation in alcoholic liver injury (20,21).
Genetic Factors
Genetic factors involved in the development and progression of ALD are demonstrated through both a genetic susceptibility to dependence and progression of liver disease. Twin studies have shown that there is a 40% to 70% heritability of substance dependence, approximately 50% heritability of alcohol dependence (22), and a concordance rate for ALD that is threefold higher in monozygotic twins compared to dizygotic twins (23).
Although it is clear that addiction is a complex phenomenon, genetic studies of alcohol dependence have focused on genes associated with neurotransmission and alcohol-metabolizing enzymes (22,24). Several studies have shown an association between polymorphisms of the GABRA2 gene (related to the inhibitory neurotransmitter GABA) and severity of alcohol withdrawal symptoms, high daily alcohol consumption, and the extent of positive reinforcement among nondependent alcohol consumers (22,24).
Furthermore, gene polymorphism of the enzymes involved in alcohol metabolism (ADH, aldehyde dehydrogenase [ALDH], and cytochrome P-450 2E1 [CYP 2E1]) has consistently been associated with alcohol dependence. Although ADH1 alleles have been shown to be protective from alcoholism, ADH2 and ADH3 alleles were associated with alcoholism in certain populations (22,24). The carriage of ALDH2*2 (base pair mutation of ALDH2) is associated with the production of low activity enzyme, conferring on its carriers an unpleasant sensation when consuming alcohol.
These polymorphic variations are associated with factors that contribute to the propensity to consume alcohol; however, they have not been shown to be associated with progression of liver disease (24,25). Genome-wide association studies looking for a genetic predictor of ALD have focused on those genes involved in the pathways of the pathogenesis of ALD: oxidative stress (manganese superoxide dismutase, glutathione S-transferase, hemochromatosis, hepatic lipid storage [peroxisome proliferator-activated receptor γ]), fibrosis (matrix metal-loproteinases), and tissue growth factors α and β. Existing smaller studies have not shown any consistent associations between these pathophysiologic pathways and the propensity to or progression of ALD (22,24,26), whereas larger studies are under way.
A genetic polymorphism of patatin-like phospholipase domain containing 3 (PNPLA3 encoding for adiponutrin, a transmembrane protein expressed in the liver and adipose tissue) has been extensively studied in nonalcoholic fatty liver disease (NAFLD) as it was found to be associated with severity of hepatic lipid accumulation and elevated liver enzymes, and this was independent of alcohol consumption, body mass index (BMI), diabetes, and ethnicity (22,24,26). Given the similarities in the pathophysiology of NAFLD and ALD, studies have also focused on this gene as a candidate gene for ALD. The single nucleotide polymorphism PNPLA3 rs738409 G was found to be strongly associated with alcohol steatohepatitis severity independent of steatosis as well as liver fibrosis and cirrhosis (22,26,27). In one study, the population attributable risk of cirrhosis in a Caucasian population with alcohol dependence in carriers of PNPLA3 rs738409 G allele was calculated at 26.6% (28). Although it appears that PNPLA rs738409 GG carriers may represent a subpopulation at high risk of progression of ALD, the mechanism of this remains unclear as is the implication it has on decision making in clinical practice and future therapies.
Other Liver Insults
Obesity
The prevalence of obesity is continuing to rise, and the disorder now affects almost 25% of adult Americans (29,30). Liver disease is one of its manifestations, and NAFLD is now recognized as a common and potentially progressive liver disease. NAFLD resembles alcoholic liver disease with respect to the pathologic appearance of liver tissue and certain mechanisms of injury including genetic associations such as with PNPLA3 (31). There is both experimental and clinical evidence of an alcohol–obesity interaction in the liver. Alcohol-fed rats develop more severe liver disease if given a high-fat diet (32). In patients who have a history of heavy alcohol consumption, high BMI has been associated with increased steatosis and is a risk factor for the development of alcohol hepatitis and cirrhosis (33–35). The effect of weight reduction on alcoholic liver disease has not been documented, but continuing fatty liver disease may explain failure to normalize liver tests in patients who abstain from alcohol.
Chronic Viral Hepatitis
Heavy alcohol use is widely recognized as a factor that is associated with advanced liver fibrosis in patients with chronic viral hepatitis, particularly hepatitis C (discussed further in the section “Hepatitis C”). The odds ratio for fibrosis exceeds 200 for heavy (more than 60 g/d) alcohol use (36). Hepatitis C has been reported to be more common in alcoholics than the general community in a number of studies (3,37), an observation attributed to but not entirely explained by an increased prevalence of injection drug use (IDU). A similar but less marked interaction between the hepatitis B virus (HBV) and the effect of alcohol on the liver has been described in heavy drinkers (38), but this interaction is not supported by all studies (39). One possible explanation for this controversy is that the older hepatitis B studies may have been confounded by unrecognized coin-fection with hepatitis C (40).
Ingestion of Hepatotoxins
Chronic alcohol consumption is associated with a range of drug interactions that may alter drug effects or increase the risk of liver injury (Fig. 74-1). Chronic ethanol consumption increases the hepatotoxicity of a number of compounds including acetaminophen, industrial solvents, anesthetic gases, isoniazid, phenylbutazone, and illicit drugs (e.g., cocaine). The induction of cytochrome P-450 2E1 (CYP 2E1) explains the increased vulnerability of the heavy drinker to these substances. CYP 2E1 oxidizes ethanol and is induced by chronic alcohol consumption. CYP 2E1 also has an extraordinary capacity to activate many xenobiotics (environmental chemicals not normally handled by the liver, such as pesticides) to highly toxic metabolites. Among patients with alcohol dependence, hepatic injury associated with acetaminophen has been described after repetitive intake for headaches (including those associated with withdrawal symptoms), dental pain, or the pain of pancreatitis. Amounts well within the accepted safe dose for the general community (2.5 to 4 g) have been incriminated as the cause of hepatic injury in patients with alcohol dependence (41,42). It is likely that the enhanced hepatotoxicity of acetaminophen after chronic ethanol consumption is caused, at least in part, by an increased microsomal production of reactive metabolite(s) of acetaminophen. Consistent with this view is the observation that, in animals chronically fed ethanol, the potentiation of acetaminophen hepatotoxicity occurs after ethanol withdrawal (43), at which time production of the toxic metabolite may be at its peak, since at that time, competition by ethanol for a common microsomal pathway has been withdrawn. Thus, maximal vulnerability to the toxicity of acetaminophen occurs immediately after cessation of drinking, when there is also the greatest need for analgesia, because of the headaches and other symptoms associated with withdrawal. This also explains the synergistic effect between acetaminophen, ethanol, and fasting (44,45), as these factors all deplete reduced glutathione (GSH). GSH provides a fundamental cellular mechanism for the scavenging of toxic free radicals. Furthermore, CYP 2E1 promotes the generation of active oxygen species that are toxic in their own right and may overwhelm the antioxidant system of the liver and other tissues with striking consequences. A similar effect may also be produced by the free hydroxyethyl radical generated from ethanol by CYP 2E1.
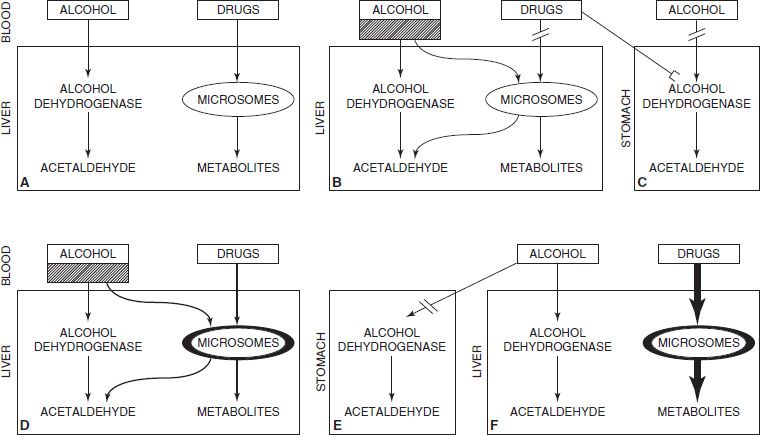
FIGURE 74-1 Schematic representation of ethanol–drug interactions. (Redrawn from Lieber CS. Alcohol and the liver: 1994 update. Gastroenterology 1994;106(4):1085–1105.)
Nutrition
For many years, alcohol per se was not thought to be hepatotoxic, and ALD was thought to be fully explained by poor nutrition. Nutritional impairment is universally present in patients with ALD and correlates with the severity of the disease. Lieber and DeCarli (46,47) showed in the baboon model that experimental alcohol administration leads to progressive liver injury, including cirrhosis, in the presence of an otherwise nutritionally adequate diet. The interaction between alcohol intake and dietary factors is complex as evidenced by studies demonstrating severe injury in animals exposed to high-dose alcohol and either high- or low-fat diets and the impact of dietary modulation on disease outcome (32,48,49).
Nutritional disorders may accelerate progression of ALD. Protein deficiency is a recognized cause for fatty liver owing to impaired apoprotein synthesis required to export lipid from hepatocytes. Choline deficiency is associated with hepatic fibrosis. Alcohol causes hyperzincuria, and zinc deficiency markedly impairs hepatic regeneration, which may be important in the regular heavy drinker (50). Vitamin A excess also leads to hepatic fibrosis. Heavy alcohol use is associated with low serum levels of vitamin A, and excessive supplementation may cause vitamin A toxicity, even with normal serum levels (5).
Pathogenesis
The pathogenesis of alcohol-related hepatitis and cirrhosis is complex and still incompletely understood. New research underscores the multiple factors involved in this disorder (26,51–60). Damage to the liver in heavy drinkers (>100 g/d) is neither predictable nor absolutely preventable. The presence of liver disease reflects the end result of interactions between the host, the toxin (alcohol), and the metabolic, immunologic, necroinflammatory, and regenerative processes.
Ethanol metabolism within the liver leads to the generation of hepatotoxic metabolites. Oxidative stress with subsequent inflammation and liver injury are recognized (56,57). This, in association with endotoxin-mediated activation of cytokine production by several cell populations within and beyond the liver (51,58,59), leads to progressive fibrogenesis. These broadly described processes interact with each other.
Alcohol and Its Metabolism
Ethanol itself contributes to liver injury. Ethanol has been shown to affect intracellular signaling pathways (60,61) by its effects on lipid membranes and its interaction with several cellular proteins, including phospholipases and adenylate cyclase.
Ethanol is metabolized to acetaldehyde and to acetate mainly via ADH and ALDH enzymes, respectively. Acetaldehyde has been shown to affect many aspects of normal cellular functioning, including DNA repair, microtubule assembly, mitochondrial respiration, fatty acid oxidation, and activation of fibrogenesis (26,55,62,63). High levels of acetaldehyde have been measured in patients with ALD, in part owing to impaired mitochondrial ALDH function (64). Acetaldehyde is highly reactive and binds covalently to normally occurring materials in blood to form compounds known as adducts (such as acetaldehyde–protein adducts and malondialdehyde–acetaldehyde adducts) (64,65).
Oxidative Stress in Alcohol-Related Liver Disease
The induction of cytochrome P-450 2E1 (CYP 2E1) generates reactive oxygen species (ROS) during oxidation of alcohol (66,67). Chronic alcohol administration induces selective depletion of mitochondrial GSH, thus diminishing cellular defense against oxidative stress (68). TNF-α release from Kupffer cells and T lymphocytes acts on hepatocytes to generate ROS in hepatic mitochondria (69).
Endotoxin-Mediated Injury
Endotoxin is a toxic lipopolysaccharide (LPS) present in the cell wall of all Gram-negative bacteria (70). Endotoxin is present in gut flora and may enter the circulation where it binds to a serum protein (lipopolysaccharide-binding protein). This complex binds to CD14, a receptor on the surface of Kupffer cells. CD14 lacks an intracellular domain, but interacts with membrane-spanning proteins called toll-like receptors (TLRs) that releases NFκB leading to up-regulation of proinflammatory cytokines (notably TNF-α) (71). TNF-α can itself increase gut permeability as well as oxidant stress perpetuating and progressing liver injury (26). This endotoxin pathway has been implicated in human ALD by several findings. Circulating levels of gut-derived endotoxin in alcoholics are increased (72). CD14 is up-regulated in Kupffer cells of alcohol-fed rats (73), and patients with ALD have high blood levels of TNF-α receptors (74). Experimental alcoholic liver injury is increased by coadministration of endotoxin (75) and is decreased with administration of antibiotics that decrease LPS production (76). However, one study has shown that rats chronically fed with alcohol develop tolerance to these effects of endotoxin (77), casting some doubt on the role of endotoxin in this disease. Nonetheless, the intermittent drinker who becomes acutely ill intermittently may still experience bouts of LPS-mediated acute liver injury.
Inflammatory Cascade
Proinflammatory cytokines promote tissue damage in experimental alcohol-related hepatitis (78,79). In human ALD, increased plasma levels of IL-1, IL-6, IL-8, IL-12, and IL-18 have been reported. Interferon-γ and TNF-α have also been implicated in human ALD. Anti-inflammatory cytokines such as IL-10 are reduced. Inflammatory factors, including IL-6, might be of fundamental importance for liver regeneration (80). Platelet-derived growth factor (PDGF) has been shown to play an important role in the repair process after acute tissue injury. PDGF is thought to promote effective necrotic tissue removal and the reconstruction of an adequate extracellular matrix network. Long-term ethanol consumption is known to inhibit the regenerative capacity of the liver, possibly via an altered transmission of growth signals through the insulin/insulin receptor substrate 1–mediated signal transduction cascade (81).
Liver Fibrosis and Regeneration
Progressive liver fibrosis underlies advanced ALD. The major source of hepatic collagen is now known to be the hepatic stellate cell (HSC, or lipocyte, Ito cell, or fat- storing cell) (82–84). Similar cells have been isolated in the pancreas (85), the kidneys, and other tissues (86). In early alcohol-related hepatic injury, fibrosis is largely pericentral (82) and characterized by accumulation of collagen types 1 and 3, proteoglycans, fibronectin, and hyaluronic acid. HSC are activated to activate and become myofibroblast-like cells that secrete collagen (82). TGF-β1 is released primarily by macrophages involved in inflammation and is the most potent profibrogenic liver cytokine. HSCs also contribute to matrix degradation during resolution of liver injury. Increasingly, liver fibrosis is seen as a potentially reversible lesion (87).
Clinical Features
Symptoms and clinical signs do not act as reliable indicators of the presence or severity of ALD as there may be no discernible symptoms even in the presence of cirrhosis. This paucity of symptoms may facilitate denial of an alcohol problem until end-stage complications occur. However, in some cases, florid clinical features do allow a confident clinical diagnosis.
ALD as a spectrum of disease can be grouped into three clinicopathologic entities that are not necessarily distinct stages and frequently coexist.
1. Alcohol-related fatty liver or steatosis is often asymptomatic and self-limited and may be completely reversible with a period of abstinence. It may be observed after several days of heavy drinking or in long-term drinking and manifests with anorexia, nausea, and right upper quadrant discomfort. The liver is enlarged (sometimes massively, with a span of 25 to 30 cm) and is soft or firm and may be tender; there are typically no signs of chronic liver disease.
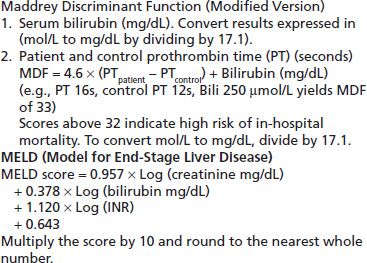
2. Alcohol-related hepatitis is classically defined by symptoms and signs of hepatitis in association with heavy alcohol use and often presents on a background of chronic liver disease. The occasional patient presents for the first time during a bout of acute alcohol-related hepatitis. Alcohol-related hepatitis presents as a spectrum of disease from mild to severe. Milder forms are most common, but severe cases, when present, carry a high short-term mortality of between 30% and 65% (88). Severe cases often present with evidence of advanced liver disease with cirrhosis and superimposed hepatic decompensation. Clinically, these patients complain of anorexia, nausea, and abdominal pain. Fever may be significant in the absence of any infective complications. They have evidence of inflammation and elevated liver enzymes (aspartate amino-transferase [AST] moderately elevated and AST–ALT ratio >2), a raised total bilirubin with jaundice, an elevated INR with bruising, encephalopathy, and often ascites. Neutrophilia is found in severe cases and, when present, should trigger a search for a septic focus, but it may simply reflect the alcohol-related liver inflammation. The severity of alcohol hepatitis can be assessed using an objective rating scale such as the Maddrey Discriminant Function (MDF) or the Model for End-Stage Liver Disease (MELD) score (89). These correlate closely with each other, and both give an indication of prognosis and may direct the use of specific treatments (Table 74-2). Patients may have few signs of chronic liver disease at first presentation, but a majority will have signs of cirrhosis at some stage of their progressive liver disorder. Unlike viral or toxic hepatitis, alcohol-related hepatitis evolves more slowly, and recovery may take many months after abstinence has been achieved.
TABLE 74-2 SCORES THAT ASSESS SHORT-TERM PROGNOSIS OF PATIENTS WITH ALCOHOLIC HEPATITIS
3. Alcohol-related cirrhosis typically presents with complications such as portal hypertension leading to variceal bleeding and/or ascites, liver failure, recurrent infections because of impaired immune function, and hepatocellular carcinoma (HCC). Occasionally, anorexia, nausea, weight loss, and general malaise are present. Alcohol-related cirrhosis is a recognized risk factor for HCC, but that risk may be less than originally thought to be the case (90). Screening for HCC is still recommended in cirrhotic patients, but the yield in pure alcohol-related disease is low (90,91).
Diagnosis and Assessment of Severity
The diagnosis of ALD rests on the history of prolonged heavy alcohol ingestion with a compatible clinical and laboratory picture. Clinical features of chronic liver disease include decreased muscle mass, palmar erythema, presence of spider nevi, hepatic lunules, loss of body hair in males and hirsutism in women, gynecomastia, splenomegaly, and caput medusa, indicating portal hypertension. In decompensated disease, there may be jaundice, encephalopathy with or without asterixis, petechial hemorrhage or ecchymoses, and ascites. Clinicians are increasingly aware of the entity of minimal hepatic encephalopathy, which alters higher center function without being associated with a metabolic flap (92). Liver enzyme tests are a sensitive marker for ALD; however, they are not specific as similar abnormalities may be observed in nonalcoholic steatohepatitis and in patients treated with medications such as anticonvulsants. The γ-glutamyl transpeptidase (γGT) level is almost always raised (93) and often exceeds 1,000 U/L in alcohol-related hepatitis. The transaminases are only moderately elevated. Levels above 500 U/L suggest an additional disorder such as acetaminophen ingestion, viral hepatitis, or liver ischemia.
The AST exceeds the alanine aminotransferase (ALT) level in most cases, and a ratio of 2:1 AST–ALT is typical. Possible explanations for this observation are that AST is a mitochondrial enzyme and alcoholic injury selectively injures mitochondria. In addition, AST is also found in other tissues subject to alcohol injury including skeletal muscle and heart. If the ALT exceeds the AST, chronic hepatitis C, acetaminophen ingestion, or other causes for hepatocellular injury should be considered.
As liver disease severity increases, falling albumin, rising bilirubin, and increasing serum globulin concentrations are seen. In cirrhosis and severe alcohol-related hepatitis, INR levels are prolonged, platelet counts fall, and there may be a macrocytic anemia with spur cells in the most severe cases. In liver failure from these conditions, blood glucose levels may fall due to loss of glycogen stores and failure of gluconeogenesis. Undiagnosed, this can be fatal.
During initial assessment for ALD, other contributing factors to developing chronic liver disease and cirrhosis should be routinely considered. Factors include ingestion of hepatotoxic drugs including herbal preparations and acetaminophen, diabetes mellitus and metabolic syndrome, hepatitis B and C infection, and iron overload. Additional investigations are restricted to atypical cases or those that fail to resolve with abstinence from alcohol. Other explanations for liver disease, including autoimmune hepatitis, Wilson disease, alpha-1-antitrypsin deficiency, and cholestatic liver disease including primary biliary cirrhosis, may need to be considered in specific cases.
Imaging of the abdomen with abdominal ultrasound, computed tomography scan, or magnetic resonance imaging has a role in establishing the presence of chronic liver disease and its complications (fatty change, portal hypertension, HCC); however, imaging is of limited value in establishing the etiology. Abdominal imaging will also allow exclusion of other pathologies that may explain abnormal liver function and enzyme tests. The role of elastography in the evaluation of liver fibrosis is being defined by many studies, and it may well prove to have a role in the assessment of patients with all forms of liver disease (94). The technology is still not approved by the FDA (95).
Once cirrhosis is suspected, referral for a diagnostic endoscopy is regarded as essential to document the presence or absence of varices. If they are present and meet appropriate requirements, prophylactic therapy is indicated (96).
Role of Liver Biopsy
Liver biopsy has been used for decades to provide information regarding the diagnosis and severity of liver disorders. In ALD, biopsy can be of use to find secondary coexisting pathology as well as establishing severity of liver disease (95). Nevertheless, the practice of biopsying the majority of patients with any form of significant liver injury has diminished owing to improvements in noninvasive imaging and diagnostic testing and as patients have become more resistant to the procedure knowing its complications and short falls. Biopsy is also subject to sampling error, and the use of the procedure is restricted now to those patients with complex or incompletely understood disease and in those being considered for liver transplantation (97,98). The morbidity of liver biopsy is 0.5%, and the mortality is 0.01% (99), and these risks generally outweigh the benefit of obtaining tissue pathology. Where clinical uncertainty persists, biopsy should always be considered. In life-threatening cases where urgent liver transplantation is being considered, vitamin K or fresh frozen plasma may be required, to reverse coagulopathy to accomplish biopsies. Transjugular biopsy is validated as a safe procedure in coagulopathic patients (100) but is not widely available and sometimes yields insufficient tissue for diagnosis.
It has been argued that demonstration of the severity of liver damage by biopsy might motivate ambivalent alcohol-dependent patients to adhere more closely to management protocols. However, this has never been validated by clinical studies, and routine use of biopsy could not be justified on this basis.
Treatment
Treatment of Causative Factors
The treatment of ALD rests upon avoidance of further alcohol consumption. Other interventions are reserved for those with particularly severe disease or who are unable to maintain abstinence. There is considerable evidence that survival is increased by maintaining abstinence (101,102). The improvement with abstinence is so consistent that the γGT falls with an apparent half-life of 26 days (103). Failure to do so suggests continuing alcohol consumption or occasionally another coexisting liver disease. Advanced cirrhosis does not resolve with abstinence, indicating that it is an irreversible lesion, but the activity is reduced. Many very ill patients make striking improvements, often returning to compensated cirrhosis. In practice, it is never too late to stop drinking.
The first issue is to define what level of alcohol consumption to recommend for the patient with liver disease. Those with true moderate to severe alcohol use disorder or severe liver disease should be given clear advice to remain abstinent long term. However, many patients have only minor abnormalities in liver enzyme tests without clinical or investigational evidence of cirrhosis. A typical recommendation is a 6-week period of abstinence followed by repeat liver tests. If these normalize and the patient wishes to resume drinking, consumption within recommended lower risk levels may be guided by the results of further liver tests in those who can control their alcohol use. Continuing follow-up in a primary care setting is important, as the major causes of death in mild ALD are extrahepatic problems related to alcohol abuse such as suicide. Another problem for the generalist physician is that many patients with alcohol-induced disorders decline referral to a specialist treatment service. This places a responsibility on internists and other primary care physicians to develop brief counseling skills including motivational interviewing. At a minimum, primary carers can provide feedback concerning medical progress, and this can influence drinking behavior.
Pharmacotherapies to address alcohol use disorders must be used with particular caution in the presence of serious liver disease. A recent study of the use of baclofen in the treatment of alcohol dependence in patients with cirrhosis has provided evidence of very positive benefit (104). Subsequent studies have not replicated this findings, which is unfortunate because of the potential benefit of the lack of liver toxicity of baclofen. Disulfiram is contraindicated in advanced liver disease owing to recognized hepatotoxicity. Another issue is the safety of acamprosate and naltrexone in patients with significant liver disease. Acamprosate does not accumulate even in severe liver disease, as the drug is excreted unchanged in the urine and is not metabolized. According to the manufacturers, acamprosate is contraindicated in severe decompensated (Childs C) liver disease and may exacerbate encephalopathy, but even in that setting, the risks of treatment should be balanced against the risks of continuing alcohol consumption, and there are no published reports of an adverse effect on liver function. Naltrexone is associated with dose-dependent hepatotoxicity (typically at supratherapeutic doses of 300 mg/d), but reactions are most unusual at the standard dose of 50 mg/d. In two studies, liver enzyme tests improved in alcoholics, with no cases of clinically evident hepatotoxicity, indicating the therapeutic effect to reduce alcohol consumption exceeded the potential hepatotoxic effect (105,106). Extended-release injectable naltrexone is a long-acting depot naltrexone product that has been shown to be effective for treatment of alcohol dependence, and a recent report did not detect any hepatotoxicity (107). Nalmefene is a second-generation orally active opiate receptor antagonist that has been reported to have similar efficacy to naltrexone in the treatment of alcohol dependence (108) without reported hepatotoxicity. It remains unapproved for use in the United States or Australia at this time. Clinicians working with alcohol-dependent patients are increasingly using these agents to reduce risks of return to drinking, and with careful monitoring of liver tests, these drugs can be safely used in many cirrhotic patients.
Managing Acute Alcoholic Hepatitis
Treatment of patients who present with alcohol-related hepatitis includes management of decompensated liver disease and the accompanying complications. Nutritional support with parenteral and enteral feeding improves nutritional status, but does not improve survival (109).
Selected cases of alcoholic hepatitis may respond to corticosteroids, but this remains controversial as individual studies and meta-analyses have shown different outcomes (110–112). Currently, the American Association for the Study of Liver Disease practice guidelines recommend prednisolone (40 mg/d for 28 days) for patients presenting with severe alcohol-related hepatitis (MDF >32; see Table 74-2) with or without hepatic encephalopathy and lacking contraindications to steroid use (88,95). Widespread use of corticosteroids has been limited by the knowledge that they may exacerbate sepsis, a common complication of severe liver disease. It is important to acknowledge that unusual infections may complicate alcohol-related disease, so specific tests may need to be requested in those in whom routine cultures fail to identify an infective agent. Infections such as tuberculosis and fungal infections including cryptococcosis need to be considered where patients fail to respond to standard therapy (113). Pentoxifylline (400 mg thrice daily), a phosphodiesterase inhibitor with many effects including decreasing serum levels of TNF-α, has also been reported to reduce the mortality of alcohol-related hepatitis, especially in patients with hepatorenal syndrome (114). Other agents have been trialed; however, none have shown any consistent survival benefit when compared with either placebo or corticosteroids. TNF-α is involved in alcoholic hepatitis, but treatment of this disease with inhibitors has not yielded any survival benefit despite promising early results (115). The trial using etanercept showed a worse 6-month survival compared with placebo (116).
Anabolic–androgenic steroids, which increase muscle mass in healthy subjects, did not show any survival benefit (117), nor have studies using antioxidants (vitamin E, silymarin) (118,119).
Treatment of Alcohol-Induced Cirrhosis
In cases where the disease is particularly severe or does not resolve with abstinence, several therapies have been trialed but have not shown mortality benefit. Propylthiouracil (120–122), colchicine (123,124), and oxandrolone (117,125) have been evaluated for the treatment of ALD, but subsequent studies failed to reproduce beneficial effects of these agents. Some agents that can be viewed as “super-nutrients” have been found to be effective in nonhuman primates. These include S-adenosylmethionine for the treatment of early aspects of alcohol-induced liver injury (126) and polyunsaturated lecithin for the prevention of fibrosis (127,128). There are some data showing that both agents are effective in humans. S-adenosylmethionine treatment led to a significantly lower mortality in patients with Childs class B cirrhosis (129); however, a more recent Cochrane Review did not show any benefit in all-cause mortality, liver-related mortality, or liver transplant (130). Polyenylphosphatidylcholine is derived from soybeans and comprises a mixture of polyunsaturated phosphatidylcholines, about half of which is dilinoleoylphosphatidylcholine. A large Veterans’ Affairs Cooperative Study evaluated this treatment but did not identify any delay in the progression to cirrhosis over the 2 years of the study, though in parallel, the participants substantially reduced their alcohol intake (131). New therapeutic targets derived from the advances in our understanding of the pathophysiology of ALD (132) include:
CXC chemokines (133,134) involved in neutrophil attraction to the liver in AH
IL-22-STAT3 pathway that controls bacterial infection homeostasis and tissue repair (135)
Complement activation that is involved in ethanol-induced liver disease in mice (136,137)
Probiotics and TLR4 antagonists that act to decrease gut microbial and LPS pathways (138,139)
Osteopontin, an extracellular matrix protein that was found to be up-regulated in patients with alcoholic hepatitis and correlates with alcohol-related liver disease severity (140,141)
Endocannabinoids (142)
Nostrin (nitric oxide synthase traffic inducer) that regulates nitric oxide synthesis (143)
At this stage of clinical practice, no new agents are available with a proven record in modifying alcohol-related liver disease (79,132,144).
Managing Complications of Cirrhosis
The management of the complications of cirrhosis, such as ascites and bleeding, lies outside the scope of this chapter. Patients who present with signs of hepatocellular insufficiency or portal hypertension should be evaluated by a gastroenterologist or hepatologist. Salt restriction helps reduce fluid retention, which presents as edema and ascites, whereas prophylactic management of portal hypertension with agents, which lower portal flow such as beta-blockers (propranolol) and long-acting nitrates or esophageal variceal banding, alters prognosis significantly. All patients presenting with ascites for the first time or representing with evidence of deteriorating clinical status should have a diagnostic ascites tap to exclude or diagnose spontaneous bacterial peritonitis (145). Once established, ascites is treated by salt restriction, spironolactone in doses from 50mg daily up to 400mg daily, and, if necessary, furosemide and bed rest. Higher doses of spironolactone may cause painful gynecomastia in men and limits the doses able to be given. In these cases, amiloride may provide a similar diuresis. Paracentesis is effective for diuretic-resistant cases and may be offered in an outpatient setting, but some data suggest that transjugular intrahepatic portal systemic shunting may be as, or more, effective (146). Severe recurrent ascites may respond to transjugular intrahepatic portasystemic shunting or surgical shunts, thus avoiding the risks of repeated paracenteses. Variceal hemorrhage is treated by transfusion, correction of coagulopathy, nitrate or octreotide infusions, and urgent endoscopic banding. After controlling bleeding, secondary prophylaxis with variceal banding and/or beta-blockers reduces the risk of rebleeding. Spontaneous peritonitis is treated by intravenous antibiotics and prophylactic norfloxacin until ascites has resolved. Hepatic encephalopathy may be alleviated by correction of precipitating factors and by decreasing colonic production and absorption of NH3 with lactulose, a nonabsorbable disaccharide that acidifies the colon content through fermentation. Dietary protein should be adjusted to avoid both deficiency and excess as nutritional deficiencies are very common in patients with advanced liver disease. Restriction of protein can impair hepatic recovery and may not necessarily improve encephalopathy (147).
Liver Transplant
Liver transplantation is now an accepted treatment option for individuals with advanced liver disease who have stopped drinking (148), but only 5% of patients with end-stage ALD receive transplants. The procedure remains controversial despite years of experience because of ethical concerns about allocation of precious donor livers to individuals with a (mis)perceived self-induced disease. It is unreasonably simplistic to regard ALD as a “self-induced disorder.” External factors such as family, peers, and society as a whole encourage the availability and use of alcohol. Genetic factors also contribute to the risk of alcohol use disorders, and understanding of this often relapsing condition remains very poorly developed (149). The 5-year survival after transplantation for ALD is comparable to that of nonalcoholics in series from the United States, Europe, and Australia (150,151). Whereas alcoholics may be at higher risk of some posttransplant problems including malignancies possibly related to smoking, there is evidence that the rate of rejection may be lower than for non-ALD (152). Resumption of alcohol consumption remains the major concern and occurs in between 30% and 50% of survivors (153,154). Of those who return to drinking, one-third develop life-threatening alcohol-related morbidity such as pancreatitis, recurrent ALD, and noncompliance with immunosuppression resulting in graft rejection (155). These outcomes are comparable to the posttransplantation recurrence rate of other liver diseases. The rate of recurrent, problematic alcohol consumption is lower than that observed after other treatments for alcohol dependence. This may be due to careful case selection for transplantation and/or the intensity of treatment by the transplant team. Vaillant (156) showed that the prognostic factors that generally predict a favorable outcome for alcoholism treatment are provided by the liver transplant process.
The ideal candidate for transplantation accepts the etiologic role of alcohol in his or her liver disease, has ceased drinking, and has strong family supports with a stable home, employment, and enthusiasm to resume interests. Psychosocial evaluation seeks to stratify patients by their risk of relapse for alcoholism. An objective and valid assessment method is highly desirable but has not been devised. The Michigan Alcoholism Prognosis Scale (157) did not predict postoperative drinking (158), possibly as only those with high scores are transplanted. Other prognostic scales have insufficient predictive value to justify rejection of an otherwise suitable candidate (159,160). Ideal candidates for transplant will have abstained on learning of the nature of their advanced liver disease, will have supportive family members available to assist their preparation for the transplant, and will have had no significant mental health issues other than their dependence on alcohol (159,161). Individual assessment remains critical, and while duration of abstinence pretransplant remains a poor predictor of outcome, most centers still aim for a minimum of 6 months abstinence before surgery.
Alcohol-related hepatitis is generally considered a contraindication to liver transplant due to the absence of 6 months of abstinence. In acute severe presentations in younger patients, transplant has been undertaken with outcomes matching those undertaken for non–alcohol-related disease (162). Some centers are reevaluating transplantation for alcohol-related hepatitis.
VIRAL HEPATITIS
Hepatitis A
Hepatitis A is an RNA virus that is transmitted by fecal– oral contamination. In underdeveloped countries, almost all children develop IgG antibodies by the age of 10, the acute infection resulting in only clinically inapparent or mild hepatitis. With improving hygiene, the seroprevalence has fallen so that adults in the developed world are now generally susceptible to hepatitis A. The clinical consequences of hepatitis A virus (HAV) infection become increasingly severe with advancing age so that hepatitis A is now less common but often more severe than in the past. The illness does not persist as a chronic infection. Parenteral transmission of hepatitis A is rare owing to the short period of viremia but has been described (163).
The prevalence of hepatitis A IgG antibodies is high among IDUs and prison inmates in California (164) and Australia (165). Hepatitis A correlates more closely with institutionalization than sharing of injecting equipment, and vaccination of seronegative prison entrants is undertaken in some jurisdictions.
Prevention of HAV infection is achieved by hygienic precautions to prevent fecal–oral contamination, administration of immunoglobulin to household contacts of cases, and active immunization to those at risk. Hepatitis vaccine is given as two injections by intramuscular injection and is safe and effective. Accepted indications for vaccination include occupational risk, travelers (especially those to high prevalence areas), men who have sex with men, and those with chronic liver disease. Vaccination has also been recommended for IDUs (166), but is not a routine practice in most clinics. HBV vaccination is a higher priority for this group (167).
Hepatitis B
Epidemiology
HBV is the most prevalent chronic viral infection of humans, and >350,000,000 people worldwide are infected. It is readily transmitted among IDUs. Serologic evidence of past hepatitis B infection increases in prevalence with the duration of injecting drug use. Sexual exposure and injecting drug use are now the commonest associations of hepatitis B infection acquired in adults in Western societies (168). In many Western countries, more new diagnoses of HBV occur in people who have migrated from countries of medium to high HBV prevalence than in those born in the country.
Transmission
HBV is highly infectious, and blood and bodily fluids are capable of transmitting the disease. Some fluids are more infectious than others with semen, vaginal fluid, and blood being most infectious. Reports of transmission from exposure to tears and saliva have been published, but the risk is low, and breast-feeding is not discouraged. In countries of high prevalence the majority of infections are transmitted during pregnancy or at delivery (termed vertical transmission) or in the first few years of life (horizontal transmission). Programs that have targeted babies born to HBV-positive mothers have reduced transmission rates, and now, universal vaccination against HBV in many countries is slowly reducing the burden of chronic HBV (CHBV) infection.
Virology
The HBV is a DNA virus with eight genotypes and multiple subtypes. Multiple mutant forms of the virus exist, and the contribution of these to infections worldwide is still being defined. A precore mutant form, which does not produce e antigen and thus leads to no e antibody production, frequently appears as viral clearance is occurring. This form of the virus can cause infection in its own right, emphasizing the need for a full serologic evaluation of new patients. Mutations involving the surface antigen of the virus lead to forms of the virus, which can be more difficult to detect by standard assays (169). Increasingly, it is evident that many infections with the HBV virus are not detected by standard assays and these infections are known as occult HBV infection. Hepatitis C virus (HCV), for example, suppresses HBV replication and may lead to a failure to recognize dual virus infection (170). Infection with the virus is associated with the production of a nuclear covalently closed circular DNA, a template of the virus that allows infection to persist even in those who apparently clear the infection and develop anti-HBs, anti-HBc, and anti-HBe antibodies.
Outcome of Infection
Vertical transmission of HBV or horizontal transmission in early childhood results in a high percentage of CHBV infection. Over 90% of those infected early in life remain infected as the immune system fails to clear the virus. On the other hand, infection in adult life is associated with an effective immune response, and clearance of serologic markers occurs in 90% to 95% of cases.
Clinical Manifestations of Acute Hepatitis B
Testing for HBsAg, anti-HBs, and anti-HBc should be performed, and seronegative persons who remain at risk of infection should be vaccinated (Table 74-3) (171). Acute and chronic hepatitis B are diagnosed by serologic tests (HBsAg, anti-HBs, anti-HBc, HBeAg, and anti-HBe) and HBV DNA testing. In combination, these assays allow one to define the phase of the infection, risk of HCC, and need for treatment.
TABLE 74-3 RECOMMENDATIONS FOR WHO SHOULD BE TESTED FOR HBV INFECTION

The incubation period of HBV infection is 6 weeks to 6 months, the period not obviously influenced by the means of infection. Acute hepatitis B may be preceded by a transient serum sickness prodrome, with polyarthralgia, fever, malaise, urticaria, and proteinuria (172). The acute illness is characterized by anorexia, nausea, and sometimes vomiting with malaise, jaundice, pale stools, and dark urine. The infection is frequently subclinical.
Chronic Hepatitis B
Chronic hepatitis B infection is associated with progressive inflammation, which leads to the histologic changes of hepatic fibrosis, cirrhosis, and HCC and the clinical presentation with hepatic failure and portal hypertension in a significant minority. Disease severity is increased by a variety of factors including duration of infection, male sex, ethnic origin, coinfection with other hepatitis viruses or HIV, alcohol ingestion or illicit drug use, and obesity and diabetes. CHBV infection is a more aggressive liver disease than is chronic HCV infection, and 25% or more with CHBV will progress to liver failure or HCC in a 10-year period.
Liver injury is not always apparent in the chronically infected individual, and the disease is now recognized to pass through a series of phases (Fig. 74-2). Inflammation, when present, is the result of a cell-mediated response to infected hepatocytes. In chronic disease, a series of hepatitis flares in the immune clearance or immune escape phases may precede viral clearance and recovery. These flares vary in severity from subclinical through to life threatening.
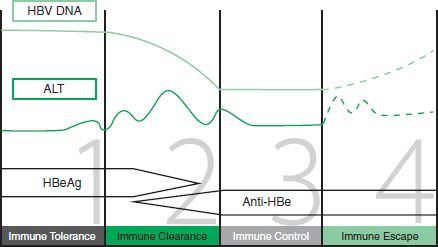
FIGURE 74-2 The four stages of chronic hepatitis B infection. (Adapted from B positive—all you want to know about hepatitis B: a guide for primary care providers. Australian Society for HIV Medicine, 2010.)
Patients with CHBV should be assessed and the phase of the disease and the HBV DNA level documented. Patients in the immune clearance or immune escape phases of the disease (with abnormal ALT levels) or those with clinical evidence of liver disease should be referred for specialist assessment and consideration of antiviral therapy. Patients with CHBV should be offered regular screening for HCC, as the risk is increased in all infected patients but especially in those with cirrhosis. Screening protocols have varied, but current evidence supports six monthly alpha-fetoprotein levels and abdominal ultrasound.
When to Treat
Any decision to treat HBV must involve patient and health care workers as treatment is usually a life time commitment with current medications. Generally, patients in the immune clearance and immune escape phases of infection are candidates for therapy. Treatment is also now considered for patients with high HBV DNA as a strategy to reduce risk of HCC. Treatment decisions are often quite complex, and patients are best managed by referral to a specialist unit for initial assessment and treatment planning. Ongoing prescribing of antiviral agents is now undertaken by primary care physicians in a number of jurisdictions.
A liver biopsy is no longer regarded as a compulsory requirement preceding treatment initiation. Ultrasound elastography, known as FibroScan, is increasingly available in some centers to provide noninvasive estimation of fibrosis and to reduce the need for biopsy.
Current Recommended First-Line Therapies
The current first-line therapies for treatment-naïve patients are the following:
■ Entecavir (ETV)—a nucleoside analogue with a high barrier to resistance (<2% at 6 years in treatment-naïve patients). Lamivudine-resistant strains develop resistance to entecavir more readily, and the drug should not be the first choice in this group of patients.
■ Tenofovir (TDF)—a nucleotide analogue with a high barrier to resistance (<1% at 5 years). This drug should be the first choice in those with lamivudine-resistant strains. This is the drug of choice in pregnancy.
Pegylated interferon (PEG-IFN) does have significant side effects but has a defined 1-year duration of therapy and the possibility of HBsAg clearance. PEG-IFN can be useful in selected younger patients, particularly women who are intending to get pregnant in the future. A response is more likely in HBeAg-positive patients with high ALT and low HBV DNA viral load.
Treatment of active chronic hepatitis B is of value in those patients with active liver inflammation and in those with very high viral load. Treatment with pegylated interferon-α (IFN-α) leads to seroconversion from eAg to eAb status in 30% to 40% compared to spontaneous seroconversion of 15%. The eAg to eAb seroconversion is associated with suppression of viral replication and decreasing hepatic inflammation (173). There is a small but continuing success rate in clearing the virus with loss of HBsAg, which rises from 5% in the first year to >10% over a 5-year follow-up. Treatment can be demonstrated to be cost-effective (174). Loss of HBeAg has been associated with improvements in liver histology and clinical outcome, but patients remain infected and infectious. Pegylated IFN-α should not be used in patients with HBV-related cirrhosis, as it may induce a flare of hepatitis and lead to hepatic decompensation. Such patients should be assessed for liver transplantation.
Oral antiviral agents are now more frequently used than interferon products because of their greater patient acceptability and high viral resistance barriers. Once commenced, these agents need to be continued for prolonged periods as dictated by the viral response to the drugs. Both entecavir and tenofovir produce a rapid and marked fall in viral load in the majority of patients. Viral clearance from serum is the goal of therapy with subsequent seroconversion from eAg positivity to anti-HBe status. In a small percentage, sAg loss occurs. Attempts to stop treatment are appropriate when the patient has had 12 months of normal liver enzymes after seroconversion to anti-HBe status. In those fortunate to have HBsAg clearance, antivirals may be ceased. Patients with HBV requiring treatment are best managed by referral to units with a special interest in HBV management.
Immunosuppressive drugs such as cancer chemotherapies are associated with increased HBV viral load and flares of hepatitis such that prophylactic treatment is recommended.
Hepatitis C Virus
Epidemiology
HCV infection is a major public health problem with over 180 million people infected worldwide (175) and approximately 3.9 million people in the United States (176). HCV remains the leading indication for liver transplantation, and it is projected that the number of people with advanced liver disease and associated HCC will continue to increase for the next two decades.
Transmission
HCV is a blood-borne virus, and in the United States, Europe, and Australia, the most common risk factor for transmission of hepatitis C infection is IDU (Table 74-4), which accounts for the bulk of incident cases (91% in Australia) (177). HCV prevalence is strongly associated with duration of injecting with an incidence of approximately 20% for each year of IDU (178). Most regular IDUs are infected with HCV, and the incidence is 100% in some populations. Measures to limit the spread of this infection appear to be making only a modest impact, and one study demonstrated an incidence of 45 per 100 life years in new injecting users in NSW Australia (179,180). The continuing high incidence appears to be related to the continuing high prevalence of sharing some component of injecting equipment including mixing spoons, filters, swabs, or tourniquets. The wider implementation of infection control procedures such as needle–syringe programs has been associated with a variable reduction of HCV transmission in new recruits to injecting (179,181,182).
TABLE 74-4 INTERPRETATION OF SEROLOGIC MARKERS FOR VIRAL HEPATITIS
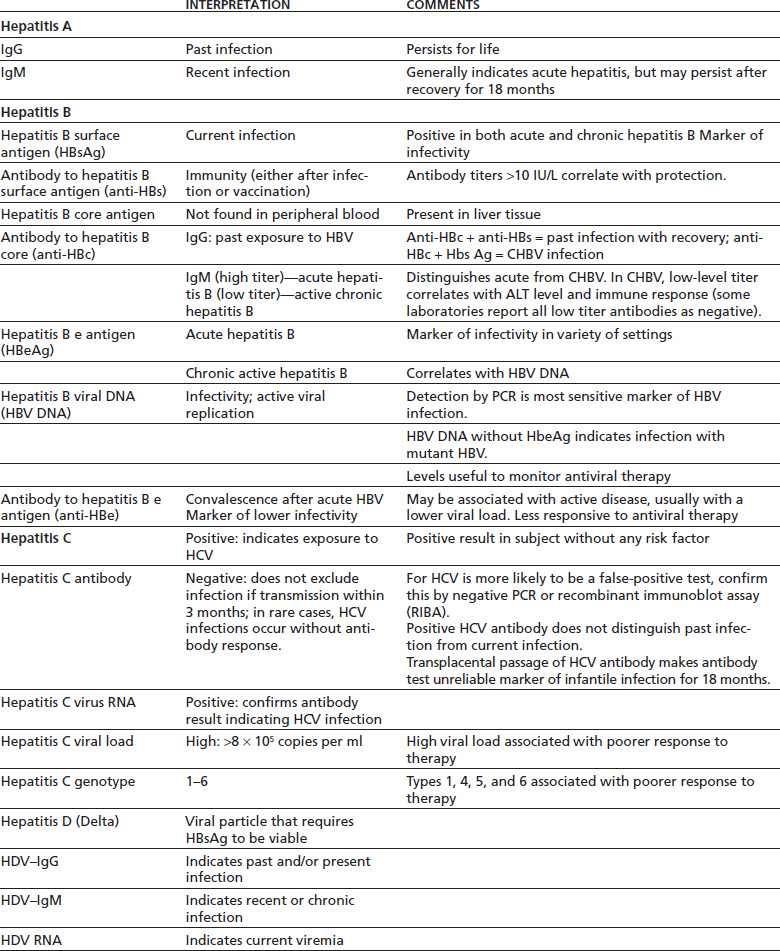
HAV, hepatitis A virus; HBV, hepatitis B virus; HCV, hepatitis C virus; PCR, polymerase chain reaction (sensitive molecular diagnostic procedure that can detect minute amounts of specific DNA or RNA).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


