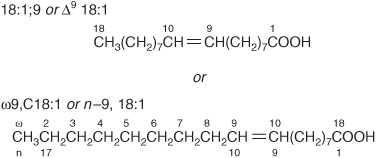15
Lipids of Physiologic Significance
Kathleen M. Botham, PhD, DSc & Peter A. Mayes, PhD, DSc
OBJECTIVES
After studying this chapter, you should be able to:
![]() Define simple and complex lipids and identify the lipid classes in each group.
Define simple and complex lipids and identify the lipid classes in each group.
![]() Indicate the structure of saturated and unsaturated fatty acids, explain how the chain length and degree of unsaturation influence their melting point, give examples, and explain the nomenclature.
Indicate the structure of saturated and unsaturated fatty acids, explain how the chain length and degree of unsaturation influence their melting point, give examples, and explain the nomenclature.
![]() Understand the difference between cis and trans carbon-carbon double bonds.
Understand the difference between cis and trans carbon-carbon double bonds.
![]() Describe how eicosanoids are formed by modification of the structure of unsaturated fatty acids; identify the various eicosanoid classes and indicate their functions.
Describe how eicosanoids are formed by modification of the structure of unsaturated fatty acids; identify the various eicosanoid classes and indicate their functions.
![]() Outline the general structure of triacylglycerols and indicate their function.
Outline the general structure of triacylglycerols and indicate their function.
![]() Outline the general structure of phospholipids and glycosphingolipids and indicate the functions of the different classes.
Outline the general structure of phospholipids and glycosphingolipids and indicate the functions of the different classes.
![]() Appreciate the importance of cholesterol as the precursor of many biologically important steroids, including steroid hormones, bile acids, and vitamins D.
Appreciate the importance of cholesterol as the precursor of many biologically important steroids, including steroid hormones, bile acids, and vitamins D.
![]() Recognize the cyclic nucleus common to all steroids and explain the difference between the “chair” and “boat” forms of the six-carbon rings and that the rings may be either cis or trans in relation to each other, making many stereoisomers possible.
Recognize the cyclic nucleus common to all steroids and explain the difference between the “chair” and “boat” forms of the six-carbon rings and that the rings may be either cis or trans in relation to each other, making many stereoisomers possible.
![]() Explain why free radicals are damaging to tissues and identify the three stages in the chain reaction of lipid peroxidation that produces them continuously.
Explain why free radicals are damaging to tissues and identify the three stages in the chain reaction of lipid peroxidation that produces them continuously.
![]() Understand how antioxidants protect lipids from peroxidation by either inhibiting chain initiation or breaking the chain and give physiological and nonphysiological examples.
Understand how antioxidants protect lipids from peroxidation by either inhibiting chain initiation or breaking the chain and give physiological and nonphysiological examples.
![]() Understand that many lipid molecules are amphipathic, having both hydrophobic and hydrophilic groups in their structure, and explain how this influences their behavior in an aqueous environment and enables certain classes, including phospholipids, sphingolipids, and cholesterol, to form the basic structure of biologic membranes.
Understand that many lipid molecules are amphipathic, having both hydrophobic and hydrophilic groups in their structure, and explain how this influences their behavior in an aqueous environment and enables certain classes, including phospholipids, sphingolipids, and cholesterol, to form the basic structure of biologic membranes.
BIOMEDICAL IMPORTANCE
The lipids are a heterogeneous group of compounds, including fats, oils, steroids, waxes, and related compounds, that are related more by their physical than by their chemical properties. They have the common property of being (1) relatively insoluble in water and (2) soluble in nonpolar solvents such as ether and chloroform. They are important dietary constituents not only because of their high energy value, but also because fat-soluble vitamins and essential fatty acids are contained in the fat of natural foods. Fat is stored in adipose tissue, where it also serves as a thermal insulator in the subcutaneous tissues and around certain organs. Nonpolar lipids act as electrical insulators, allowing rapid propagation of depolarization waves along myelinated nerves. Combinations of lipid and protein (lipoproteins) serve as the means of transporting lipids in the blood. Lipids have essential roles in nutrition and health and knowledge of lipid biochemistry is necessary for the understanding of many important biomedical conditions, including obesity, diabetes mellitus, and atherosclerosis.
LIPIDS ARE CLASSIFIED AS SIMPLE OR COMPLEX
1. Simple lipids: Esters of fatty acids with various alcohols.
a. Fats: Esters of fatty acids with glycerol. Oils are fats in the liquid state.
b. Waxes: Esters of fatty acids with higher molecular weight monohydric alcohols.
2. Complex lipids: Esters of fatty acids containing groups in addition to an alcohol and a fatty acid.
a. Phospholipids: Lipids containing, in addition to fatty acids and an alcohol, a phosphoric acid residue. They frequently have nitrogen-containing bases and other substituents, for example, in glycerophospholipids the alcohol is glycerol and in sphingophospholipids the alcohol is sphingosine.
b. Glycolipids (glycosphingolipids): Lipids containing a fatty acid, sphingosine, and carbohydrate.
c. Other complex lipids: Lipids such as sulfolipids and amino lipids. Lipoproteins may also be placed in this category.
3. Precursor and derived lipids: These include fatty acids, glycerol, steroids, other alcohols, fatty aldehydes, ketone bodies (Chapter 22), hydrocarbons, lipid-soluble vitamins, and hormones.
Because they are uncharged, acylglycerols (glycerides), cholesterol, and cholesteryl esters are termed neutral lipids.
FATTY ACIDS ARE ALIPHATIC CARBOXYLIC ACIDS
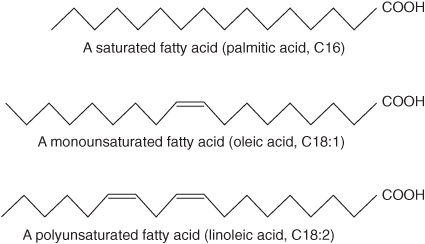
Fatty acids occur in the body mainly as esters in natural fats and oils, but are found in the unesterified form as free fatty acids, a transport form in the plasma. Fatty acids that occur in natural fats usually contain an even number of carbon atoms. The chain may be saturated (containing no double bonds) or unsaturated (containing one or more double bonds) (Figure 15–1).
FIGURE 15–1 Fatty acids.
Fatty Acids Are Named after Corresponding Hydrocarbons
The most frequently used systematic nomenclature names the fatty acid after the hydrocarbon with the same number and arrangement of carbon atoms, with -oic being substituted for the final -e (Genevan system). Thus, saturated acids end in -anoic, for example, octanoic acid, and unsaturated acids with double bonds end in -enoic, for example, octadecenoic acid (oleic acid).
Carbon atoms are numbered from the carboxyl carbon (carbon no. 1). The carbon atoms adjacent to the carboxyl carbon (nos. 2, 3, and 4) are also known as the α, β, and γ carbons, respectively, and the terminal methyl carbon is known as the ω- or n-carbon.
Various conventions use Δ for indicating the number and position of the double bonds (Figure 15–2); for example, Δ9 indicates a double bond between carbons 9 and 10 of the fatty acid; ω9 indicates a double bond on the ninth carbon counting from the ω-carbon. In animals, additional double bonds are introduced only between the existing double bond (eg, ω9, ω6, or ω3) and the carboxyl carbon, leading to three series of fatty acids known as the ω9, ω6, and ω3 families, respectively.
FIGURE 15–2 Oleic acid. n – 9 is equivalent to ω9.
Saturated Fatty Acids Contain No Double Bonds
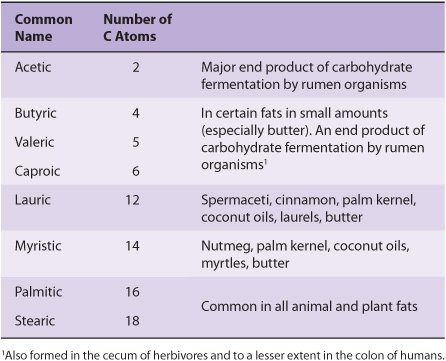
Saturated fatty acids may be envisaged as based on acetic acid (CH3—COOH) as the first member of the series in which—CH2—is progressively added between the terminal CH3—and—COOH groups. Examples are shown in Table 15-1. Other higher members of the series are known to occur, particularly in waxes. A few branched-chain fatty acids have also been isolated from both plant and animal sources.
TABLE 15–1 Saturated Fatty Acids
Unsaturated Fatty Acids Contain One or More Double Bonds
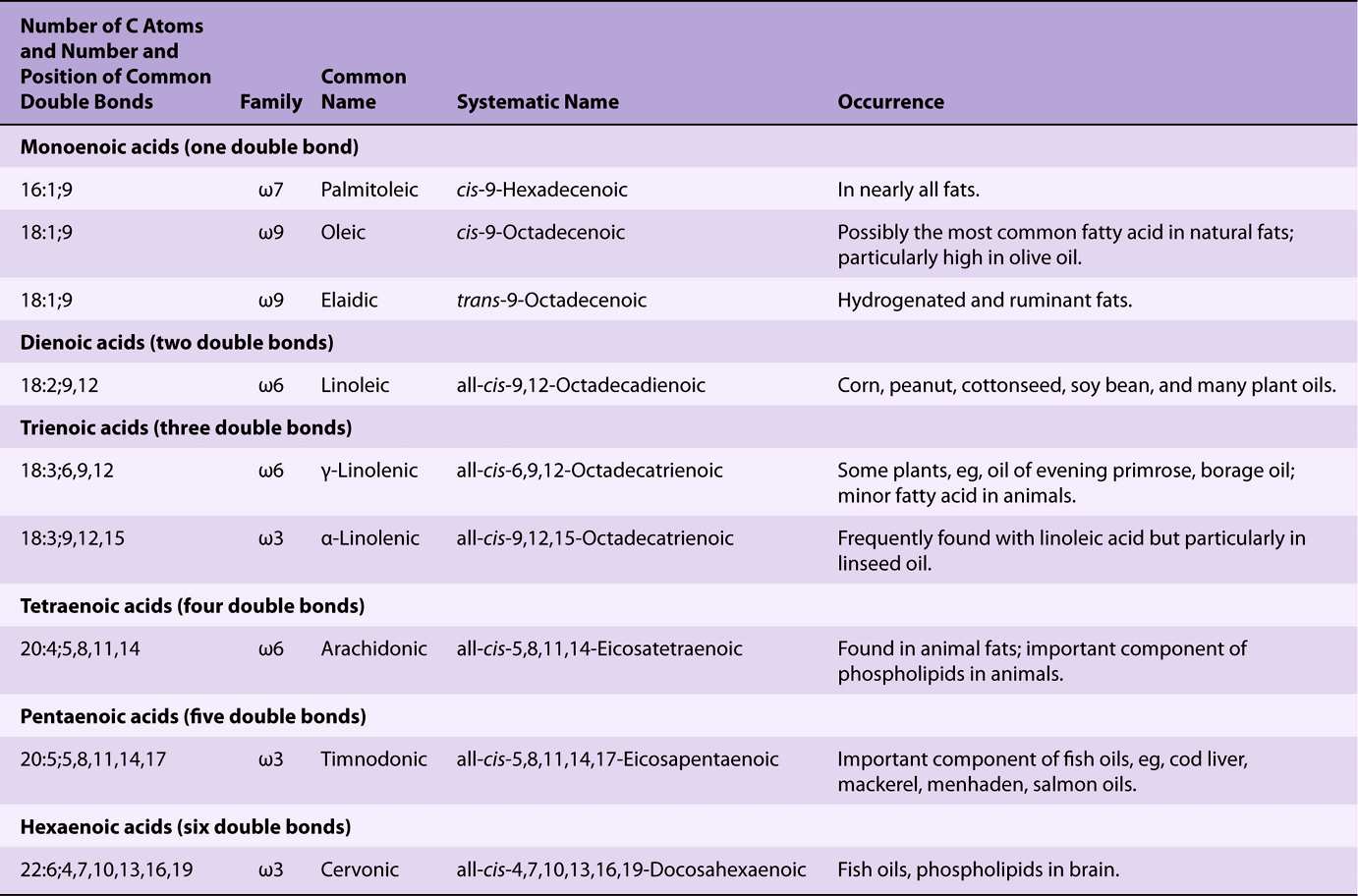
Unsaturated fatty acids (Figure 15–1, Table 15-2) may be further subdivided as follows:
TABLE 15–2 Unsaturated Fatty Acids of Physiologic and Nutritional Significance
1. Monounsaturated (monoethenoid, monoenoic) acids, containing one double bond.
2. Polyunsaturated (polyethenoid, polyenoic) acids, containing two or more double bonds.
3. Eicosanoids: These compounds, derived from eicosa (20-carbon) polyenoic fatty acids, comprise the prostanoids, leukotrienes (LTs), and lipoxins (LXs). Prostanoids include prostaglandins (PGs), prostacyclins (PGIs), and thromboxanes (TXs).
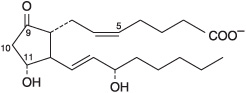
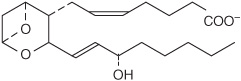
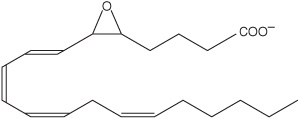
Prostaglandins exist in virtually every mammalian tissue, acting as local hormones; they have important physiologic and pharmacologic activities. They are synthesized in vivo by cyclization of the center of the carbon chain of 20-carbon (eicosanoic) polyunsaturated fatty acids (eg, arachidonic acid) to form a cyclopentane ring (Figure 15–3). A related series of compounds, the thromboxanes, have the cyclopentane ring interrupted with an oxygen atom (oxane ring) (Figure 15–4). Three different eicosanoic fatty acids give rise to three groups of eicosanoids characterized by the number of double bonds in the side chains, for example, PG1, PG2, and PG3. Different substituent groups attached to the rings give rise to series of prostaglandins and thromboxanes, labeled A, B, etc—for example, the “E” type of prostaglandin (as in PGE2) has a keto group in position 9, whereas the “F” type has a hydroxyl group in this position. The leukotrienes and lipoxins (Figure 15–5) are a third group of eicosanoid derivatives formed via the lipoxygenase pathway (Figure 23–11). They are characterized by the presence of three or four conjugated double bonds, respectively. Leukotrienes cause bronchoconstriction as well as being potent proinflammatory agents, and play a part in asthma.
FIGURE 15–3 Prostaglandin E2 (PGE2).
FIGURE 15–4 Thromboxane A2 (TXA2).
FIGURE 15–5 Leukotriene A4 (LTA4).
Most Naturally Occurring Unsaturated Fatty Acids Have cis Double Bonds
The carbon chains of saturated fatty acids form a zigzag pattern when extended at low temperatures (Figure 15–1). At higher temperatures, some bonds rotate, causing chain shortening, which explains why biomembranes become thinner with increases in temperature. A type of geometric isomerism occurs in unsaturated fatty acids, depending on the orientation of atoms or groups around the axes of double bonds, which do not allow rotation. If the acyl chains are on the same side of the bond, it is cis-, as in oleic acid; if on opposite sides, it is trans-, as in elaidic acid, the trans isomer of oleic acid (Figure 15–6). Double bonds in naturally occurring unsaturated long-chain fatty acids are nearly all in the cis configuration, the molecules being “bent” 120° at the double bond. Thus, oleic acid has an L shape, whereas elaidic acid remains “straight.” Increase in the number of cis double bonds in a fatty acid leads to a variety of possible spatial configurations of the molecule—for example, arachidonic acid, with four cis
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree