25
Lipid Transport & Storage
Kathleen M. Botham, PhD, DSc & Peter A. Mayes, PhD, DSc
OBJECTIVES
After studying this chapter, you should be able to:
![]() Identify the four major groups of plasma lipoproteins and the four major lipid classes they carry.
Identify the four major groups of plasma lipoproteins and the four major lipid classes they carry.
![]() Illustrate the structure of a lipoprotein particle.
Illustrate the structure of a lipoprotein particle.
![]() Indicate the major types of apolipoprotein found in the different lipoprotein classes.
Indicate the major types of apolipoprotein found in the different lipoprotein classes.
![]() Explain that triacylglycerol is carried from the intestine (after intake from the diet) to the liver in chylomicrons and from the liver to extrahepatic tissues in very low density lipoprotein (VLDL), and these particles are synthesized in intestinal and liver cells, respectively, by similar processes.
Explain that triacylglycerol is carried from the intestine (after intake from the diet) to the liver in chylomicrons and from the liver to extrahepatic tissues in very low density lipoprotein (VLDL), and these particles are synthesized in intestinal and liver cells, respectively, by similar processes.
![]() Illustrate the processes by which chylomicrons are metabolized by lipases to form chylomicron remnants, which are then removed from the circulation by the liver.
Illustrate the processes by which chylomicrons are metabolized by lipases to form chylomicron remnants, which are then removed from the circulation by the liver.
![]() Explain how VLDL is metabolized by lipases to VLDL remnants (also called intermediate-density lipoprotein (IDL)) which may be cleared by the liver or converted to low-density lipoprotein (LDL), which functions to deliver cholesterol from the liver to extrahepatic tissues and is taken up via the LDL (apoB100,E) receptor.
Explain how VLDL is metabolized by lipases to VLDL remnants (also called intermediate-density lipoprotein (IDL)) which may be cleared by the liver or converted to low-density lipoprotein (LDL), which functions to deliver cholesterol from the liver to extrahepatic tissues and is taken up via the LDL (apoB100,E) receptor.
![]() Explain how high-density lipoprotein (HDL), which returns cholesterol from extrahepatic tissues to the liver in reverse cholesterol transport, is synthesized, indicate the mechanisms by which it accepts cholesterol from tissues, and show how it is metabolized in the HDL cycle.
Explain how high-density lipoprotein (HDL), which returns cholesterol from extrahepatic tissues to the liver in reverse cholesterol transport, is synthesized, indicate the mechanisms by which it accepts cholesterol from tissues, and show how it is metabolized in the HDL cycle.
![]() Understand how the liver plays a central role in lipid transport and metabolism and how hepatic VLDL secretion is regulated by the diet and hormones.
Understand how the liver plays a central role in lipid transport and metabolism and how hepatic VLDL secretion is regulated by the diet and hormones.
![]() Be aware of the roles of LDL and HDL in promoting and retarding, respectively, the development of atherosclerosis.
Be aware of the roles of LDL and HDL in promoting and retarding, respectively, the development of atherosclerosis.
![]() Indicate the causes of alcoholic and nonalcoholic fatty liver disease.
Indicate the causes of alcoholic and nonalcoholic fatty liver disease.
![]() Appreciate that adipose tissue is the main store of triacylglycerol in the body and explain the processes by which fatty acids are released and how they are regulated.
Appreciate that adipose tissue is the main store of triacylglycerol in the body and explain the processes by which fatty acids are released and how they are regulated.
![]() Understand the role of brown adipose tissue in the generation of body heat.
Understand the role of brown adipose tissue in the generation of body heat.
BIOMEDICAL IMPORTANCE
Fat absorbed from the diet and lipids synthesized by the liver and adipose tissue must be transported between the various tissues and organs for utilization and storage. Since lipids are insoluble in water, the problem of how to transport them in the aqueous blood plasma is solved by associating nonpolar lipids (triacylglycerol and cholesteryl esters) with amphipathic lipids (phospholipids and cholesterol) and proteins to make water-miscible lipoproteins.
In a meal-eating omnivore such as the human, excess calories are ingested in the anabolic phase of the feeding cycle, followed by a period of negative caloric balance when the organism draws upon its carbohydrate and fat stores. Lipoproteins mediate this cycle by transporting lipids from the intestines as chylomicrons—and from the liver as very low density lipoproteins (VLDL)—to most tissues for oxidation and to adipose tissue for storage. Lipid is mobilized from adipose tissue as free fatty acids (FFA) bound to serum albumin. Abnormalities of lipoprotein metabolism cause various hypo- or hyperlipoproteinemias. The most common of these is in diabetes mellitus, where insulin deficiency causes excessive mobilization of FFA and underutilization of chylomicrons and VLDL, leading to hypertriacylglycerolemia. Most other pathologic conditions affecting lipid transport are due primarily to inherited defects, some of which cause hypercholesterolemia and premature atherosclerosis (Table 26-1). Obesity—particularly abdominal obesity—is a risk factor for increased mortality, hypertension, type 2 diabetes mellitus, hyperlipidemia, hyperglycemia, and various endocrine dysfunctions.
LIPIDS ARE TRANSPORTED IN THE PLASMA AS LIPOPROTEINS
Four Major Lipid Classes Are Present in Lipoproteins
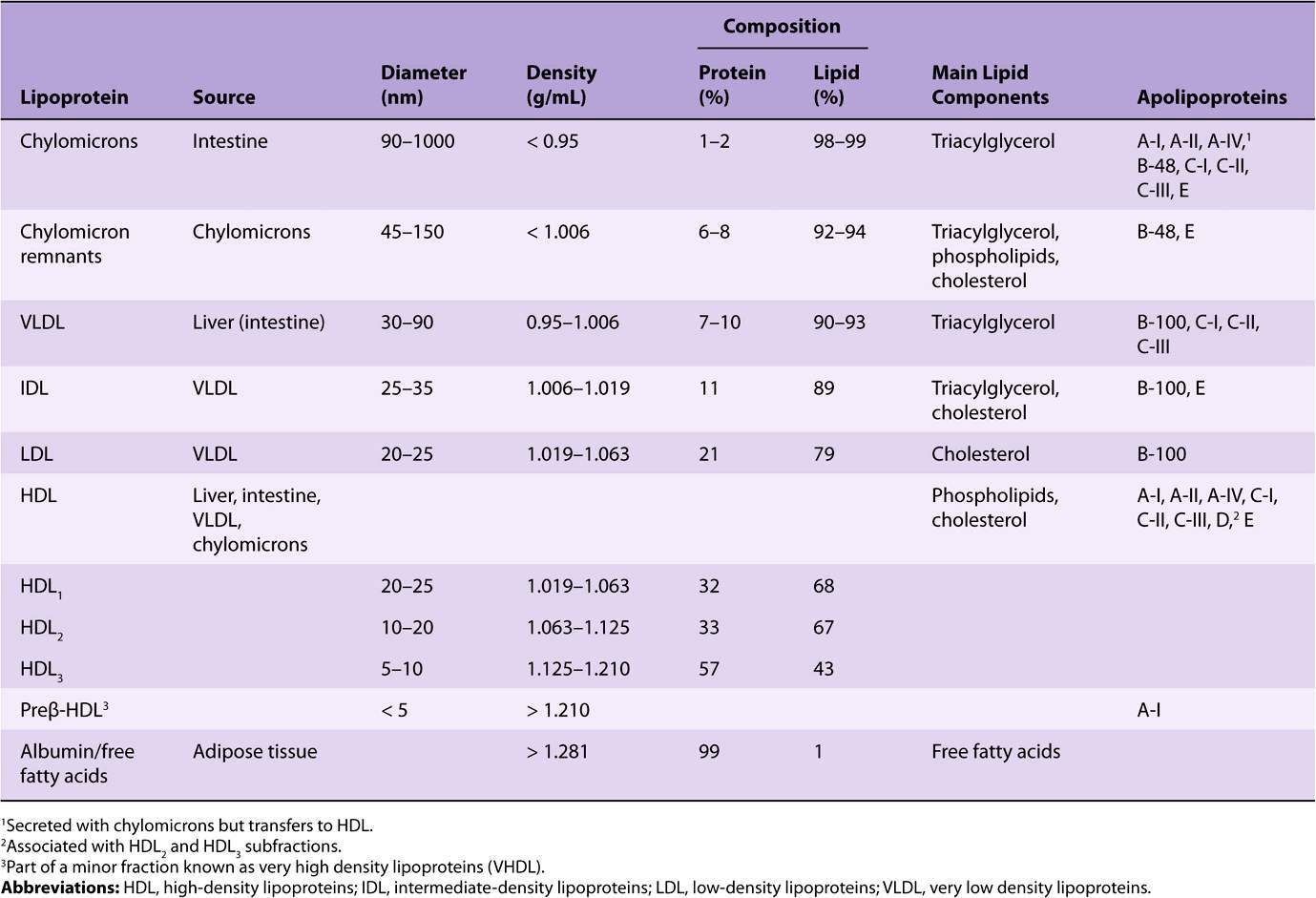
Plasma lipids consist of triacylglycerols (16%), phospholipids (30%), cholesterol (14%), and cholesteryl esters (36%) and a much smaller fraction of unesterified long-chain fatty acids (free fatty acids or FFA) (4%). This latter fraction, the FFA, is metabolically the most active of the plasma lipids.
Four Major Groups of Plasma Lipoproteins Have Been Identified
Since fat is less dense than water, the density of a lipoprotein decreases as the proportion of lipid to protein increases (Table 25-1). Four major groups of lipoproteins have been identified that are important physiologically and in clinical diagnosis. These are (1) chylomicrons, derived from intestinal absorption of triacylglycerol and other lipids; (2) very low density lipoproteins (VLDL, or pre-β-lipoproteins), derived from the liver for the export of triacylglycerol; (3) low-density lipoproteins (LDL, or β-lipoproteins), representing a final stage in the catabolism of VLDL; and (4) high-density lipoproteins (HDL, or α-lipoproteins), involved in cholesterol transport and also in VLDL and chylomicron metabolism. Triacylglycerol is the predominant lipid in chylomicrons and VLDL, whereas cholesterol and phospholipid are the predominant lipids in LDL and HDL, respectively (Table 25-1). Lipo-proteins may be separated according to their electrophoretic properties into α-, β-, and pre-β-lipoproteins.
TABLE 25–1 Composition of the Lipoproteins in Plasma of Humans
Lipoproteins Consist of a Nonpolar Core & a Single Surface Layer of Amphipathic Lipids
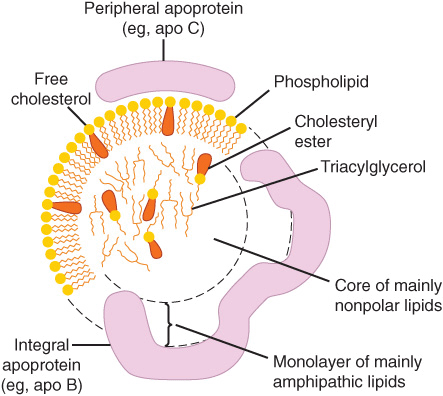
The nonpolar lipid core consists of mainly triacylglycerol and cholesteryl ester and is surrounded by a single surface layer of amphipathic phospholipid and cholesterol molecules (Figure 25–1). These are oriented so that their polar groups face outward to the aqueous medium, as in the cell membrane (Chapter 15). The protein moiety of a lipoprotein is known as an apolipoprotein or apoprotein, constituting nearly 70% of some HDL and as little as 1% of chylomicrons. Some apolipoproteins are integral and cannot be removed, whereas others are free to transfer to other lipoproteins.
FIGURE 25–1 Generalized structure of a plasma lipoprotein. The similarities with the structure of the plasma membrane are to be noted. Small amounts of cholesteryl ester and triacylglycerol are found in the surface layer and a little free cholesterol in the core.
The Distribution of Apolipoproteins Characterizes the Lipoprotein
One or more apolipoproteins (proteins or polypeptides) are present in each lipoprotein. The major apolipoproteins of HDL (α-lipoprotein) are designated A (Table 25-1). The main apolipoprotein of LDL (β-lipoprotein) is apolipoprotein B (B-100), which is found also in VLDL. Chylomicrons contain a truncated form of apo B (B-48) that is synthesized in the intestine, while B-100 is synthesized in the liver. Apo B-100 is one of the longest single polypeptide chains known, having 4536 amino acids and a molecular mass of 550,000 Da. Apo B-48 (48% of B-100) is formed after transcription of the apo B-100 gene by the introduction of a stop signal into the mRNA transcript by an RNA editing enzyme. Apo C-I, C-II, and C-III are smaller polypeptides (molecular mass 7000-9000 Da) freely transferable between several different lipoproteins. Apo E, found in VLDL, HDL, chylomicrons, and chylomicron remnants, is also freely transferable; it accounts for 5-10% of total VLDL apolipoproteins in normal subjects.
Apolipoproteins carry out several roles: (1) they can form part of the structure of the lipoprotein, eg, apo B; (2) they are enzyme cofactors, eg, C-II for lipoprotein lipase, A-I for lecithin:cholesterol acyltransferase, or enzyme inhibitors, eg, apo A-II and apo C-III for lipoprotein lipase, apo C-I for cholesteryl ester transfer protein; and (3) they act as ligands for interaction with lipoprotein receptors in tissues, eg, apo B-100 and apo E for the LDL receptor, apo E for the LDL-receptor-related protein (LRP), which has been identified as the remnant receptor, and apo A-I for the HDL receptor. The functions of apo A-IV and apo D, however, are not yet clearly defined, although apo D is believed to be an important factor in human neurodegenerative disorders.
FREE FATTY ACIDS ARE RAPIDLY METABOLIZED
The FFA (also termed nonesterified fatty acids or unesterified fatty acids) arise in the plasma from the breakdown of triacylglycerol in adipose tissue or as a result of the action of lipoprotein lipase on the plasma triacylglycerols. They are found in combination with albumin, a very effective solubilizer, in concentrations varying between 0.1 and 2.0 μeq/mL of plasma. Levels are low in the fully fed condition and rise to 0.7-0.8 μeq/mL in the starved state. In uncontrolled diabetes mellitus, the level may rise to as much as 2 μeq/mL.
FFA are removed from the blood extremely rapidly and oxidized (fulfilling 25-50% of energy requirements in starvation) or esterified to form triacylglycerol in the tissues. In starvation, esterified lipids from the circulation or in the tissues are oxidized as well, particularly in heart and skeletal muscle cells, where considerable stores of lipid are to be found.
The FFA uptake by tissues is related directly to the plasma-FFA concentration, which in turn is determined by the rate of lipolysis in adipose tissue. After dissociation of the fatty acid-albumin complex at the plasma membrane, fatty acids bind to a membrane fatty acid transport protein that acts as a transmembrane cotransporter with Na+. On entering the cytosol, FFA are bound by intracellular fatty-acid-binding proteins. The role of these proteins in intracellular transport is thought to be similar to that of serum albumin in extracellular transport of long-chain fatty acids.
TRIACYLGLYCEROL IS TRANSPORTED FROM THE INTESTINES IN CHYLOMICRONS & FROM THE LIVER IN VERY LOW DENSITY LIPOPROTEINS
By definition, chylomicrons are found in chyle formed only by the lymphatic system draining the intestine. They are responsible for the transport of all dietary lipids into the circulation. Small quantities of VLDL are also to be found in chyle; however, most VLDL in the plasma are of hepatic origin. They are the vehicles of transport of triacylglycerol from the liver to the extrahepatic tissues.
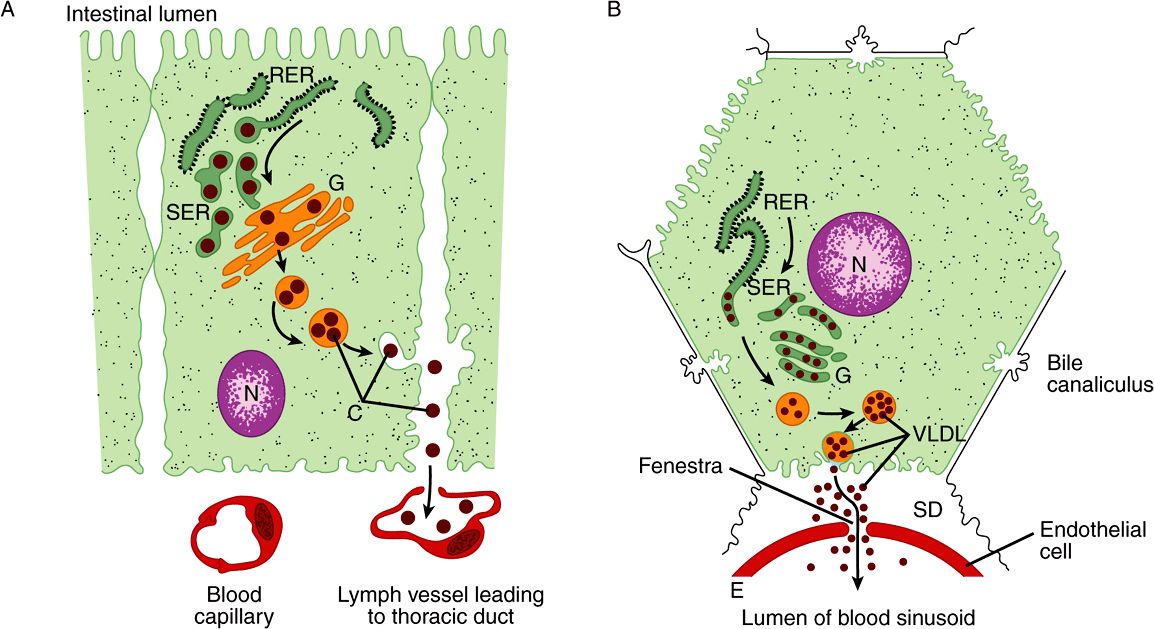
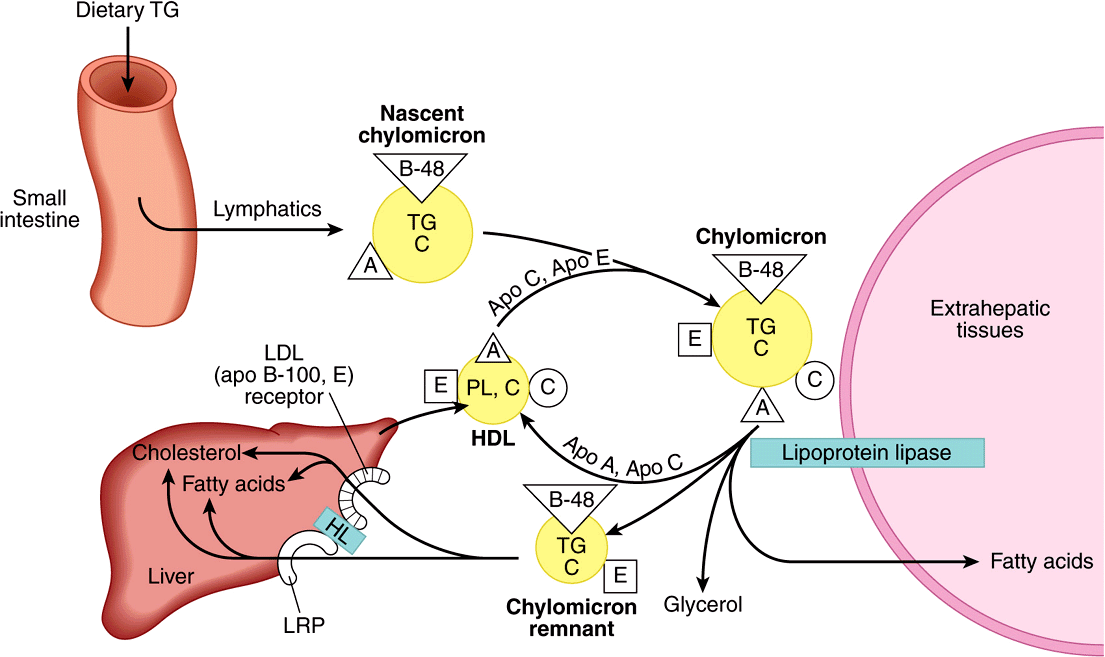
There are striking similarities in the mechanisms of formation of chylomicrons by intestinal cells and of VLDL by hepatic parenchymal cells (Figure 25–2), perhaps because—apart from the mammary gland—the intestine and liver are the only tissues from which particulate lipid is secreted. Newly secreted or “nascent” chylomicrons and VLDL contain only a small amount of apolipoproteins C and E, and the full complement is acquired from HDL in the circulation (Figures 25-3 and 25-4). Apo B, however, is an integral part of the lipoprotein particles, it is incorporated inside the cells and is essential for chylomicron and VLDL formation. In abetalipoproteinemia (a rare disease), lipoproteins containing apo B are not formed and lipid droplets accumulate in the intestine and liver.
FIGURE 25–2 The formation and secretion of (A) chylomicrons by an intestinal cell and (B) very low density lipoproteins by a hepatic cell. (C, chylomicrons; E, endothelium; G, Golgi apparatus; N, nucleus; RER, rough endoplasmic reticulum; SD, space of Disse, containing blood plasma; SER, smooth endoplasmic reticulum; VLDL, very low density lipoproteins.) Apolipoprotein B, synthesized in the RER, is incorporated into particles with triacylglycerol, cholesterol, and phospholipids in the SER. After the addition of carbohydrate residues in G, they are released from the cell by reverse pinocytosis. Chylomicrons pass into the lymphatic system. VLDL are secreted into the space of Disse and then into the hepatic sinusoids through fenestrae in the endothelial lining.
FIGURE 25–3 Metabolic fate of chylomicrons. (A, apolipoprotein A; B-48, apolipoprotein B-48; C, apolipoprotein C; C, cholesterol and cholesteryl ester; E, apolipoprotein E; HDL, high-density lipoprotein; HL, hepatic lipase; LRP, LDL-receptor-related protein; PL, phospholipid; TG, triacylglycerol.) Only the predominant lipids are shown.
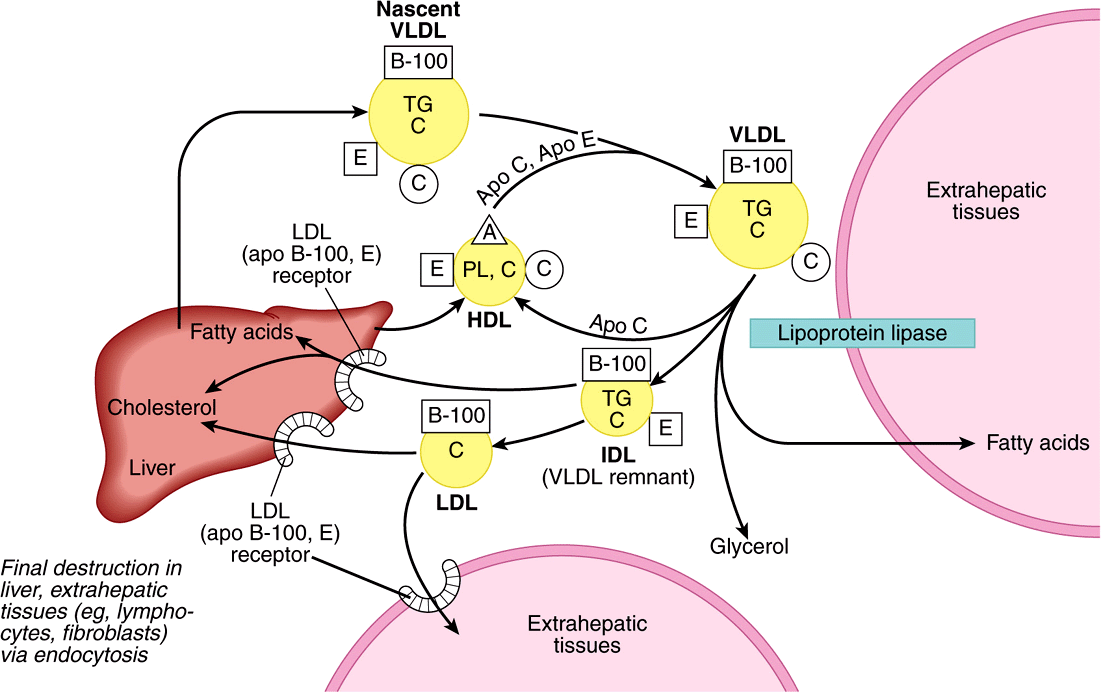
FIGURE 25–4 Metabolic fate of very low density lipoproteins (VLDL) and production of low-density lipoproteins (LDL). (A, apolipoprotein A; B-100, apolipoprotein B-100; C, apolipoprotein C; C, cholesterol and cholesteryl ester; E, apolipoprotein E; HDL, high-density lipoprotein; IDL, intermediate-density lipoprotein; PL, phospholipid; TG, triacylglycerol.) Only the predominant lipids are shown. It is possible that some IDL is also metabolized via the low density lipoproten receptor-related protein (LRP.)
A more detailed account of the factors controlling hepatic VLDL secretion is given below.
CHYLOMICRONS & VERY LOW DENSITY LIPOPROTEINS ARE RAPIDLY CATABOLIZED
The clearance of chylomicrons from the blood is rapid, the half-time of disappearance being under 1 h in humans. Larger particles are catabolized more quickly than smaller ones. Fatty acids originating from chylomicron triacylglycerol are delivered mainly to adipose tissue, heart, and muscle (80%), while ~20% goes to the liver. However, the liver does not metabolize native chylomicrons or VLDL significantly; thus, the fatty acids in the liver must be secondary to their metabolism in extrahepatic tissues.
Triacylglycerols of Chylomicrons & VLDL Are Hydrolyzed by Lipoprotein Lipase
Lipoprotein lipase is located on the walls of blood capillaries, anchored to the endothelium by negatively charged proteoglycan chains of heparan sulfate. It has been found in heart, adipose tissue, spleen, lung, renal medulla, aorta, diaphragm, and lactating mammary gland, although it is not active in adult liver. It is not normally found in blood; however, following injection of heparin, lipoprotein lipase is released from its heparan sulfate binding sites into the circulation. Hepatic lipase is bound to the sinusoidal surface of liver cells and is also released by heparin. This enzyme, however, does not react readily with chylomicrons or VLDL but is involved in chylomicron remnant and HDL metabolism.
Both phospholipids and apo C-II are required as cofactors for lipoprotein lipase activity, while apo A-II and apo C-III act as inhibitors. Hydrolysis takes place while the lipoproteins are attached to the enzyme on the endothelium. Triacylglycerol is hydrolyzed progressively through a diacylglycerol to a monoacylglycerol and finally to FFA plus glycerol. Some of the released FFA return to the circulation, attached to albumin, but the bulk is transported into the tissue (Figures 25-3 and 25-4). Heart lipoprotein lipase has a low K for triacylglycerol, about one-tenth of that for the enzyme in adipose tissue. This enables the delivery of fatty acids from triacylglycerol to be redirected from adipose tissue to the heart in the starved state
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree