Laparoscopic Gastric Bypass
Philip R. Schauer
Stacy A. Brethauer
Introduction
The field of bariatric surgery continues to grow at a rapid pace. Worldwide, over 350,000 bariatric procedures are performed yearly and over 90% of these are performed laparoscopically. Despite an overall increase in laparoscopic adjustable gastric banding procedures in recent years, nearly half of all bariatric operations performed worldwide are gastric bypass (Table 1). Europe, in particular, has seen a recent decline in the number of banding procedures in favor of gastric bypass. As bariatric surgery becomes an integral component of many general surgery programs and as the metabolic, economic, and mortality benefits of Roux-en-Y gastric bypass continue to be proven, many surgeons will continue to seek advanced training to perform this procedure.
History
Gastric bypass was first performed as a weight loss procedure in 1967 by Mason and Ito after they recognized that patients undergoing partial gastrectomy had difficulty gaining weight. The original operation consisted of a 150-mL gastric pouch and a loop gastrojejunostomy. Over the last four decades, the operation has been modified significantly to reduce the incidence of bile reflux and to create a small divided gastric pouch. Some surgeons augment this restrictive component by placing a fixed band around the pouch.
Wittgrove and Clark demonstrated the feasibility of the laparoscopic Roux-en-Y gastric bypass (LRYGB) in 1994. A small series of studies in the late 1990s followed this initial study and reported weight loss and comorbidity resolution similar to the open approach. Since that time there have been many large studies demonstrating the safety and efficacy of LRYGB and several randomized trials demonstrating the benefits of the laparoscopic approach with regard to postoperative pulmonary function, pain, and wound complications. Over the last 5 years, the physiologic effects of gastric bypass, particularly those related to improvement in diabetes, have been the focus of much research and discussion. The term “metabolic surgery” has been added to our vernacular to emphasize the important effects that gastric bypass and other bariatric procedures have on diabetes, other metabolic comorbidities, and cardiovascular risk.
Table 1 Bariatric Procedures Performed Worldwide | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||
Candidates for Laparoscopic Gastric Bypass and Patient Selection
The 1991 National Institutes of Health (NIH) Consensus Conference on Gastric Surgery for Severe Obesity provided criteria for performing bariatric surgery. Appropriate candidates have a body mass index (BMI) ≥40 kg/m2 or a BMI ≥35 kg/m2 with obesity-related comorbidity. These criteria provide an arbitrary starting point for the evaluation of the obese patient, but by no means guarantee that he or she will be a good candidate for bariatric surgery. Bariatric surgery patients should undergo an extensive preoperative evaluation prior to being scheduled for a procedure. The work-up should address the patient’s known comorbidities and should attempt to uncover occult comorbidities such as coronary artery disease, sleep apnea, and obesity hypoventilation syndrome. A multidisciplinary approach is required to fully evaluate bariatric surgery candidates. The team should include nutritionists, psychologists and psychiatrists, and medical subspecialists that can optimize the patient’s cardiovascular, pulmonary, and endocrine status prior to surgery. Family and social support is required for a successful outcome as well. Patients with ongoing substance abuse or uncontrolled psychiatric disorders are not candidates for bariatric surgery.
Patients who are preparing for bariatric surgery must be fully informed about the risks and benefits for all of the available surgical options. Currently, there is no evidence-based algorithm available that can predict which operation best suits an individual patient. Rather, the patient’s preference (usually related to how risk averse they are) in combination with their medical history and the surgeon’s experience drive the decision-making process. The follow-up requirements (adjustments for bands, additional supplements for bypass procedures) must be taken into consideration to achieve a successful outcome with any bariatric procedure.
There are several factors that put patients at higher risk for complications. Male gender, age over 55, BMI >45, hypertension, and high risk of pulmonary embolism (PE, previous thrombosis, pulmonary embolus, inferior vena cava filter, right heart failure, and obesity hypoventilation) have all been identified as factors that contribute to mortality after gastric bypass.
Mechanism of Action
The standard Roux-en-Y gastric bypass is a restrictive procedure (very small capacity of the gastric pouch) combined with a limited bypass of the proximal bowel. The standard Roux limb length is 75 to 150 cm and the biliopancreatic limb length is typically 30 to 50 cm beyond the ligament of Treitz. This leaves several meters of “common channel” bowel length beyond the jejuno-jejunostomy for nutrient absorption and this configuration does not result in malabsorption of macronutrients under normal circumstances. Some micronutrients, though, rely more on the proximal bowel for absorption (iron, vitamin B12, calcium, vitamin D, calcium) and patients can become deficient if they do not take lifelong supplementation.
Identifying the mechanisms underlying the rapid improvement of type 2 diabetes mellitus after gastric bypass has been the focus of many research efforts. Glucose metabolism improves rapidly after gastric bypass and this typically precedes weight loss. Caloric restriction in the early postoperative period certainly plays a role, but the rapid delivery of nutrients to the distal bowel results in early and exaggerated production of hormones that stimulate pancreatic beta cells (incretins) as well as increased production of gut hormones responsible for satiety. Additionally, the exclusion of nutrient flow through the proximal bowel after gastric bypass may also play a role in this incretin effect, though the specific hormones underlying this mechanism are not well defined.
Technique
Access and Exposure
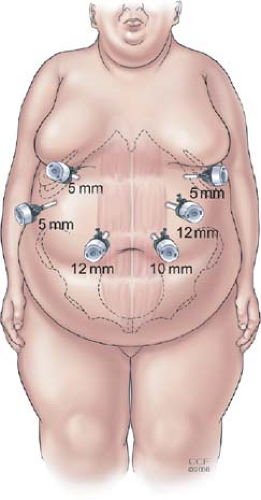
We place the patient in the supine position with the feet together on a footboard. Heavy tape is used to secure the patient’s legs to the bed above and below the knees to prevent the knees from bending when the patient is in full reverse Trendelenberg position. The operating surgeon stands on the patient’s right side and the assistant on the left. Pneumoperitoneum is established with a Veress needle through a left upper quadrant incision. Visual access to the peritoneal cavity is then obtained using a 5-mm optical viewing trocar (Endopath Xcel, Ethicon Endosurgery, Cincinnati, OH) and the remaining ports are placed under direct vision after needle localization and infiltration of local anesthetic (Fig. 1). If there are severe adhesions to the abdominal wall from a prior laparotomy, an additional 5-mm trocar is placed in the left lower quadrant to create an adequate working space for the remaining ports. A 5-mm liver retractor (Snowden-Pencer, Tucker, GA) is placed through the right lateral port and anchored to the bed with a self-retaining device. A Nathanson liver retractor can also be used in the subxiphoid position and, for very large patients with extremely large or floppy left hepatic lobes, both retractor systems can be used simultaneously to achieve adequate exposure of the gastroesophageal junction.
Jejuno-Jejunostomy and Roux Limb (Fig. 2)
The transverse colon and omentum are reflected superiorly to the upper abdomen and the ligament of Treitz is identified. It is helpful to have the assistant hold the mesocolon upward with a grasper to maintain adequate exposure during creation of the jejuno-jejunostomy. The proximal jejunum is placed in a “C” configuration toward the camera to help orient the proximal and distal segments. The jejunum is then divided 30 to 50 cm distal to the ligament of Treitz using a white load of the linear cutting stapler (2.5-mm staples). The mesentery of the jejunum is further divided with additional staple firings or an energy source to provide sufficient length of mesentery for tension-free passage of the Roux limb to the gastric pouch. The Roux limb is marked by sewing a Penrose drain or stitch to the corner.
The Roux limb is measured distally from the Penrose drain for a distance of 150 cm. The bowel should be straightened (not stretched) against a rigid measuring device such as a marked grasper to determine the proper Roux limb length. Once the appropriate length is measured, a suture is placed to approximate the biliopancreatic limb and the Roux limb side by side. With the assistant holding upward on the stay suture, small adjacent enterotomies are made with the harmonic scalpel (Ethicon Endosurgery, Cincinnati, OH). It is helpful to stagger these enterotomies slightly (Roux limb enterotomy more proximal) when placing the two ends of the stapler into the respective lumens to keep the stapler cartridge in the lumen while placing the anvil on the other side. A side-to-side jejuno-jejunostomy is then created with one firing of the 60-mm stapler and the remaining common enterotomy is then closed with another firing of the linear stapler. Care is taken not to narrow the lumen of the Roux limb with this final firing of the stapler. If there is concern about creating a tight “bottleneck” with the enterotomy closure where the Roux limb enters the anastomosis, it can be closed with a running suture. Once the anastomosis is completed, a reinforcing “crotch” stitch is placed at the distal corner of the staple line and another stitch is placed between the stapled end of the biliopancreatic limb and the Roux limb (“Brolin stitch”) to keep the Roux limb from kinking as it enters the anastomosis. The mesenteric defect between the biliopancreatic limb mesentery and Roux limb mesentery is then closed with a running nonabsorbable suture.
The omentum is then divided with the ultrasonic shears down to the midportion of the transverse colon to provide a “valley” for the antecolic Roux limb. In some cases, the gastro-colic fat is very thick and this can be divided as well to avoid tension on the gastrojejunostomy. The Roux limb is passed upward between the leaves of the divided omentum to the gastric pouch in the antecolic and antegastric position. The attached Penrose drain or stitch provides an atraumatic handle for this maneuver. The Roux limb can also be placed in the retrocolic position through a defect created in the mesocolon (Fig. 3). Some surgeons perform this technique routinely and others use it only as needed to minimize tension on the Roux limb. When the retrocolic technique is used, the mesocolic defect and space between the mesocolon and Roux limb mesentery (Peterson’s space) should be closed with a nonabsorbable suture as these are two potential sites for internal hernia formation.
Gastric Pouch (Fig. 4A)
The patient is placed in steep reverse Trendelenberg position and exposure of the gastroesophageal junction is obtained. The assistant grabs the omentum along the upper stomach and retracts it downward to expose the gastric fundus and flatten out the stomach. This can sometimes be facilitated by rolling the tissue in the grasper in a clockwise direction. The gastric dissection is started by creating a window in the pars flaccida of the gastrohepatic ligament. Once the retrogastric space is entered and the left gastric artery is identified, the
lesser omentum and descending branches of the left gastric vessels are divided 1 or 2 cm below the left gastric artery. This is completed using either a linear stapler or an energy source. Prior to stapling the stomach, reconfirm with the anesthesia team that all gastric tubes and temperature probes have been removed from the patient’s nose and mouth. One horizontal firing of the linear stapler with a blue load (3.5-mm staples) is then completed. Using an articulating linear stapler, two or three vertical firings are directed to the angle of His to create a 15- to 20-mL vertically oriented gastric pouch. A small amount of gastric serosa should be visible on the left side of the stapler for this last firing to avoid potential leaks at the gastroesophageal junction. Gentle dissection of the gastric pouch off of the left diaphragmatic crus improves mobility of the pouch and minimizes tension on the gastrojejunal anastomosis.
lesser omentum and descending branches of the left gastric vessels are divided 1 or 2 cm below the left gastric artery. This is completed using either a linear stapler or an energy source. Prior to stapling the stomach, reconfirm with the anesthesia team that all gastric tubes and temperature probes have been removed from the patient’s nose and mouth. One horizontal firing of the linear stapler with a blue load (3.5-mm staples) is then completed. Using an articulating linear stapler, two or three vertical firings are directed to the angle of His to create a 15- to 20-mL vertically oriented gastric pouch. A small amount of gastric serosa should be visible on the left side of the stapler for this last firing to avoid potential leaks at the gastroesophageal junction. Gentle dissection of the gastric pouch off of the left diaphragmatic crus improves mobility of the pouch and minimizes tension on the gastrojejunal anastomosis.
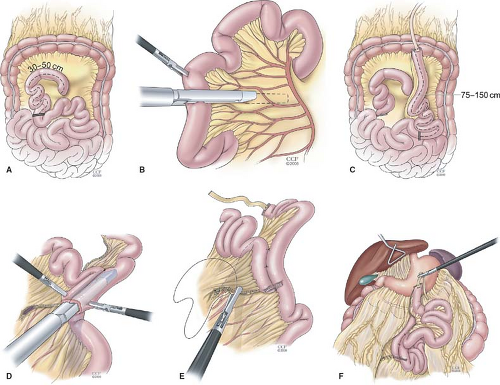 Fig. 2. Technique of laparoscopic Roux-en-Y gastric bypass. Creation of the Roux limb. A: The jejunum is placed in a “C” configuration and divided 30 to 50 cm from the ligament of Treitz with a linear stapler. B: The mesentery is also divided with two firings of the stapler or energy source. C: The distal segment (Roux limb) is marked with a Penrose drain and a 150-cm length of jejunum is measured. D: A side-to-side, functional end-to-end jejunojejunostomy is created with the linear stapler. E: The common opening is closed with a linear stapler and the mesenteric defect is closed with running nonabsorbable suture. F: After the Roux limb is created, the greater omentum is divided and the Roux limb is placed in the antecolic, antegastric position.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|