Laparoscopic Gastrectomy
Aurora Dawn Pryor
Laparoscopic approaches to the stomach are becoming increasingly common. Although much of this push comes from bariatric surgery, there has been significant crossover into other disease processes as well. Newer techniques incorporating endoscopic and single site approaches are minimizing the invasiveness of gastric procedures. With high-volume laparoscopic centers minimizing length of stay and maximizing return to work, the morbidity of gastric procedures has been diminished.
Gastric resections are performed for a variety of different diagnoses. Despite the underlying pathology, there are some basic tenets to gastric resection that can be utilized for most patients. Gastric resections can be divided into four major subsets: local/wedge, proximal, distal, and total gastrectomy. This chapter will review gastric pathology requiring resection and minimally invasive surgical techniques.
Peptic Ulcer Disease
Surgical management of peptic ulcer disease has metamorphosed with the advent of proton pump inhibitor (PPI) therapy and elucidation of the role of Helicobacter pylori in gastric pathology. While gastric resections for ulcer disease were formerly a mainstay in the practice of general surgery, such procedures are now rare. Most patients are treated with 6 weeks of PPI therapy and eradication of H. pylori. This combination of therapies is effective in treating the vast majority of ulcers. However, indications do remain for surgical therapy for ulcerative disease. The major indication is failure of medical management presenting as intractable ulceration after a 3-month medical trial or issues such as perforation or bleeding while on therapy. Longer-term complications of ulcers, such as gastric outlet obstruction or malignancy, remain reasons for surgery as well. An additional relative indication for surgery is a patient with complicated ulcer disease and poor access to care or unlikely follow-up.
The choice of surgical procedure is based on the indication. Simple Graham (omental) patch closure can be performed for pyloric channel or duodenal perforation. Vagotomy and pyloroplasty offer a definitive approach to a bleeding pyloric channel or duodenal ulcer. Wedge gastrectomy results in management of perforated gastric ulcers, while providing adequate tissue to evaluate for underlying malignancy. Antrectomy, usually in conjunction with vagotomy, is most commonly used for patients with gastric outlet obstruction from chronic ulcer disease. Vagotomy and antrectomy can also be used for definitive management of a perforated pyloric channel ulcer in a stable patient with minimal soilage.
Gastrointestinal Stromal Tumors
Gastrointestinal stromal tumors (GISTs) are mesenchymal neoplasms originating from the cells of Cajal. Sixty percent of these tumors are found in the stomach. The diagnosis is suspected with any submucosal mass, and confirmed in most cases with reactivity to KIT (CD117) and/or DOG1 (Discovered on GISTI). Many GISTs are incidentally found, but some present as a source of bleeding due to rupture. The malignant potential increases with both size and mitotic index, with tumors <2 cm in diameter and with ≤5 mitoses per 50 HPF considered very low risk. Surgery is the mainstay of treatment for localized GISTs amenable to resection.
Surgical resection for GISTs involves complete margin-negative resection with an intact pseudocapsule. Lymphadenectomy is not required. These features make laparoscopic management particularly appealing for most general surgeons. Details of the resection are dependent on the location of the tumor. The technical focus is to obtain negative margins without causing obstruction. If margins are positive, reresection should be considered. Locally advanced tumors can be considered for neoadjuvant therapy with either imatinib mesylate or sunitinib malate, both tyrosine kinase inhibitors. Following resection, patients with high-risk tumors should be considered for adjuvant therapy with these agents as well.
Pancreatic rests and adenomas may present preoperatively as suspected GISTs. Although they have low malignant potential, they are usually managed like GISTs unless a benign diagnosis can be confirmed.
Lymphoma
Gastric lymphoma has also transitioned to medical from surgical management with delineation of the role of H. pylori. Gastric MALT (mucosa-associated lymphoid tissue)-type lymphoma is often due to chronic H. pylori infection, and is frequently treated by eradication of H. pylori. Management of diffuse large B-cell lymphoma (DLBCL) has also transitioned to primarily chemoradiation from surgical resection. The incidence of bleeding or perforation during chemoradiation is only 2%. Patients with lymphoma refractive to medical management or with complications such as bleeding, perforation, or obstruction are the small subset still considered for surgery. The type of resection in these patients is determined by the specific complication or resection to negative margins as possible.
Carcinoids
Gastric neuroendocrine tumors are an unusual cause of gastric pathology. These lesions can be benign carcinoid tumors or neuroendocrine carcinomas (NECs). This determination can usually be made based on hematoxylin- and eosin-stained biopsies. Some carcinoid lesions may be associated with atrophic gastritis, pernicious anemia, or elevated gastrin levels. Most small lesions confirmed to be benign can be removed with endoscopic or laparoscopic wedge techniques. Some advocate surveillance for benign lesions 10 to 20 mm or smaller. Many suggest that multifocal carcinoids should be resected with antrectomy. NECs should be managed with more radical resection, often total gastrectomy with lymphadenectomy.
Adenocarcinoma
Gastric adenocarcinoma has a variable presentation based on geography. In the Western Pacific region, the incidence of gastric adenocarcinoma is higher; however, increased screening protocols exist and the disease is usually identified at an earlier stage and with enhanced survival than typically seen in Europe or the Americas. Interestingly, Asian Americans in North America present with disease that more closely resembles symptoms observed in patients the Western Pacific Regions. H. pylori eradication programs have also been associated with a decline in the incidence of gastric carcinoma worldwide.
Patients in North America are usually identified at endoscopy performed for symptoms. As many as 80% to 90% of these patients may have locally advanced or metastatic disease at presentation. Therefore, thorough staging should be performed before planning surgery. Proximal gastric lesions arising within 5 cm of and involving the GE junction are staged and managed as esophageal cancers. Computed tomography (CT) scanning and endoscopic ultrasound are helpful preoperative staging modalities. Based on the results of a landmark 2006 study by Cunningham and colleagues, patients with gastric or gastroesophageal (GE) adenocarcinoma should receive preoperative chemotherapy to improve progression-free and overall survival.
The surgical approach is geared at obtaining an R0 resection, with negative margins and no evidence of residual disease whenever possible. Endoscopic mucosal resection and/or endoscopic submucosal dissection may be considered for mucosal-based lesions less than 2 cm without evidence of nodal disease. Although this practice is established in Japan and Korea, its use in the United States is in its infancy.
Laparoscopic management of a patient with gastric cancer should begin with a diagnostic laparoscopy. Based on data from the Norwegian Stomach Cancer Trial, patients with even stage III or IV disease may have a survival benefit with gastric resection. The only subsets that did not benefit from resection had extensive peritoneal, hepatic, or nodal spread. In the United States and Europe, D1 lymphadenectomy involving just the perigastric nodes is standard. More extensive D2 lymphadenectomy adding nodal tissue surrounding the left gastric, splenic, and hepatic arteries as well as the celiac axis, although beneficial in
Japan, has been associated with increased morbidity and mortality in Western trials. Removal of at least 15 lymph nodes is important for staging information.
Japan, has been associated with increased morbidity and mortality in Western trials. Removal of at least 15 lymph nodes is important for staging information.
Outcomes after laparoscopic versus open gastric resection are oncologically equivalent based on several studies in the setting of experienced laparoscopic surgeons and lower risk patients (ASA <3). There are benefits with length of stay, resumption of oral intake, blood loss, and analgesia use as well. Robotic surgery may facilitate D2 lymphadenectomy and is employed in some Eastern centers.
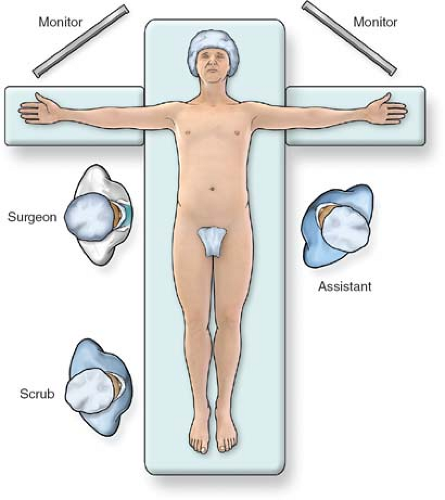 Fig. 1. Operating room setup for laparoscopic gastrectomy. The patient is placed supine on the table with arms extended. The surgeon stands to the patient’s right and the assistant is on the left. |
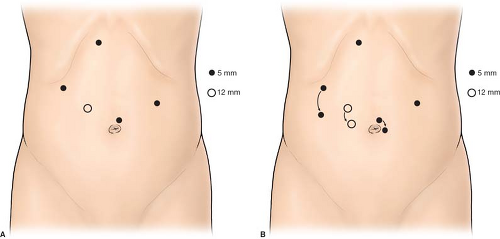 Fig. 2. Port placement for laparoscopic gastrectomy. The ports are placed higher (A) for proximal pathology or lower (B) for more distal lesions. |
For patients with advanced disease, palliative resection, even involving total gastrectomy, has improved outcomes compared with surgical bypass. This includes both quality of life and possibly survival benefits.
The setup for most laparoscopic gastric resections is standard. In our practice, the patient is placed supine with the arms extended out at the sides (Fig. 1). For most pathology, the surgeon stands at the patient’s right, with the assistant on the left. Monitors are placed over the patient’s shoulders. An endoscope should be readily available to help with identification of pathology, or to test for leak at the completion of the procedure. Ports are placed in a similar port configuration for most resections (Fig. 2). Ports are placed somewhat skewed lower and to the right for distal pathology, and higher for GE junction approaches. The procedures are generally performed in reverse Trendelenburg positioning, with the exception of transgastric approaches, or when manipulating the jejunum for a Roux limb. A liver retractor is helpful for proximal gastric exposure. This is typically placed through a subxiphoid port.
Local/Wedge Resection
The approach to wedge resection varies dramatically based on the location of the gastric pathology. The most straightforward resections are for lesions in the generous and forgiving anterior body of the stomach. Both proximal and distal lesions offer some challenges to avoid obstruction.
Anterior Body
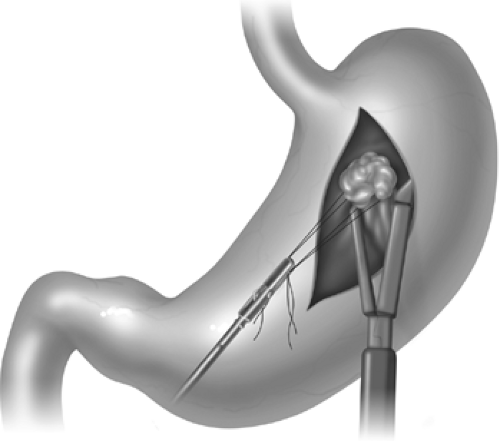
Lesions in the anterior body are relatively easy to manage. If the lesion is exophytic and can thus be visualized upon inspection, it can often be elevated with a grasper and removed by stapling it away from the remainder of the stomach with linear staplers (Fig. 3). Sutures flanking the mass can sometimes be used to assist in retraction and ensure more generous margins. If the mass is endophytic, occasionally dimpling or an endoscopic tattoo may be visible to help with localization. If these are not apparent, endoscopic identification of the pathology may be necessary. In general 2.5- to 3.5-mm staple leg lengths are used for the transaction, depending on tissue thickness. Hybrid leg lengths or buttress material may be added based on surgeon preference. Following complete transaction, the staple line should be inspected and any irregularities oversewn. It is also helpful to augment visual inspection with an endoscopic leak test: insufflation coupled with submerging the anastomosis/staple line. The goal of wedge resection is negative margins, and these should be confirmed by frozen section if not clear on gross inspection.
Posterior Body
Lesions along the greater or lesser curvatures may be addressed by skeletonizing the neurovascular arcades off of the stomach and proceeding with techniques similar to anterior body wedge resections. However, as pathology progresses to the posterior stomach, alternative approaches should be considered. The two most straightforward techniques for posterior wall lesions are (a) creation of an anterior gastrotomy coupled with eversion and wedge resection of the pathology (Fig. 4) or (b) laparoscopic transgastric approaches. With anterior gastrotomy, in addition to the wedge resection on the posterior wall, the anterior gastrotomy should be closed with staples or sutures.
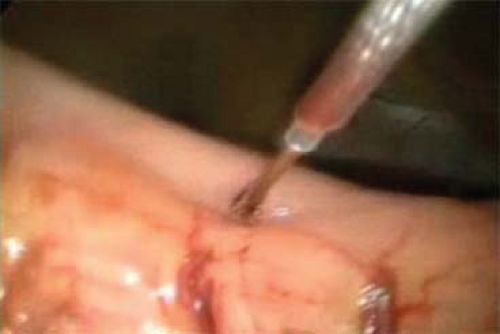 Fig. 5. Placing a transgastric laparoscopic trocar. Stay sutures are used to hold up the stomach as facilitate placement. Balloon-tipped or -flanged trocars may also be a useful adjunct. |
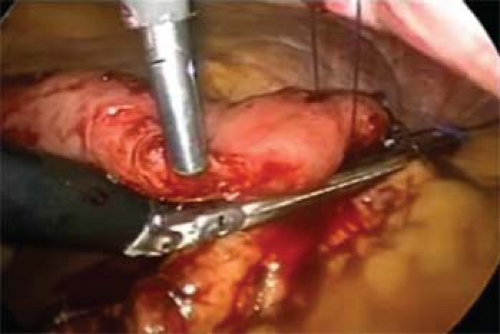
If a laparoscopic transgastric approach is utilized, ports are placed into the anterior gastric wall and the stomach itself is insufflated (Figs. 5 and 6). To set up for this, diagnostic laparoscopy is first performed using one umbilical port, and additional trocars that can be repositioned intragastrically. It is helpful to use flange or balloon-tipped trocars and to place sutures in the anterior gastric wall to minimize the risk of the ports pulling out during the procedure. Ports should be placed as far from the pathology as technically possible to give working space. In our practice, we place ports with consideration of eventual closure, away from the greater curvature vasculature and in a relatively confined, linear space. Often only two ports are adequate. Long sutures are placed through the pathology and out through the ports to aid in retraction (Fig. 7). A laparoscope or stapler can be placed alongside the retraction suture. Roticulating staple loads are then used for the wedge resection. Care must be taken to avoid narrowing the lumen near the GE junction or the pylorus. Following specimen removal, the ports are backed out of the stomach and the gastrotomies closed with staples or sutures.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree