Laparoscopic Esophagectomy
James D. Luketich
Jon O. Wee
Introduction
Esophageal resection may be a life-saving and life-enhancing procedure for those patients with early malignancy or end-stage functional disorders, such as achalasia. The open operative approaches, such as those described by Orringer and McKeown, are still the standard of care for esophageal resections. However, the morbidity and mortality associated with these procedures in most medical centers remains significant. A 10-year review of the open esophagectomy experience within the Veterans Affairs system revealed a morbidity of 50% and a mortality of 10%. Birkmeyer and associates, in an analysis of a national Medicare database, revealed that the mortality rates from esophagectomy in the United States ranged from 8% in high-volume centers to a staggering 23% in low-volume centers.
With the advent of laparoscopy and thoracoscopy, surgeons began to develop minimally invasive approaches to esophageal surgery. Initial experience with laparoscopic Nissen fundoplication formed the basis of the early esophageal surgical experience. Collard was the first to describe a thoracoscopic technique for esophageal dissection. Though multiple reports of laparoscopic-assisted esophagectomies followed, it was not until 1996, when DePaula, published his initial experience, that a totally laparoscopic esophagectomy was documented. Similarly, Swanstrom reported the technical feasibility and good short-term results in a small series of patients undergoing a totally laparoscopic esophagectomy.
One issue with minimally invasive esophagectomy is defining what constitutes a minimally invasive approach. Some surgeons have combined a thoracoscopic approach with a laparotomy, while others reported a laparoscopic abdominal component with an open thoracotomy for the chest. While these hybrid operations do fall under the umbrella of minimally invasive esophageal resection, a strict definition of minimally invasive esophageal resection avoids both a thoracotomy and a laparotomy completely. In our experience, we initially started with a laparoscopic transhiatal approach (in 15 patients) and attempted to reach our finger dissection from the neck incision. We found this to be quite difficult in most patients. We then converted to a combined thoracoscopic/laparoscopic approach with a neck anastomosis, as it afforded better visualization of the periesophageal structures especially near the main airways and subcarinal areas, was less affected by patient height and body habitus, improved our ability to do a more complete nodal dissection, and greatly improved overall visualization compared with the totally laparoscopic method. This minimally invasive three-hole (McKeown approach) operation was our procedure of choice for 6 to 8 years (approximately 500 cases). Following this extensive minimally invasive experience, we moved to a minimally invasive Ivor Lewis approach in which we start laparoscopically and complete using a right thoracoscopic approach and a high thoracic anastomosis. We prefer this approach because the avoidance of a neck dissection essentially eliminates recurrent laryngeal nerve injury. Additionally, minimally invasive Ivor Lewis resection allows a more aggressive gastric resection margin, amputation of the most proximal tip of the newly constructed gastric conduit, which is the most susceptible to ischemia and potential leak, and performance of the anastomosis with a wide view within the chest cavity.
In 2000, Nyugen et al. compared minimally invasive esophagectomy with open transthoracic and transhiatal esophagectomy. Shorter operative times, less blood loss, and shorter stays in the ICU with no increase in morbidity were documented with the minimally invasive approach as compared with the open approach. Over the past several years, several series have been published on minimally invasive resections that demonstrate comparable survival outcomes compared with large open series.
Indications for the minimally invasive approach for esophagectomy include Barrett’s esophagus with high-grade dysplasia, end-stage achalasia, esophageal strictures, and esophageal cancer. While most T4 esophageal cancers are generally not amenable to open or minimally invasive surgical approaches, cancers of all other T stages are potentially amenable to minimally invasive esophagectomy in experienced hands. Esophageal cancer that has been downstaged after neoadjuvant chemoradiation is also potentially resectable by a minimally invasive approach but, as with open operations in this setting, can be technically more difficult than nonirradiated fields. Previous thoracic and abdominal surgery is not necessarily a contraindication to minimally invasive esophagectomy depending on the extent of the previous surgery and the experience of the surgeon. The minimally invasive Ivor Lewis esophagectomy works well for most distal esophageal cancers, short-to-moderate length Barrett’s with high-grade dysplasia, and gastroesophageal junction tumors extending onto the gastric cardia. Total laparoscopic and thoracoscopic Ivor Lewis resections should not be performed for upper third esophageal cancers with significant proximal extension due to concern for adequate margins of resection. Some mid-esophageal tumors can be approached with the Ivor Lewis technique, but a very high intrathoracic anastomosis may be needed to gain a negative margin.
Laparoscopy
Before placement of the laparoscopic ports, esophagogastroscopy is performed in all patients to confirm the location of the tumor and suitability of the stomach for tubularization and as a new conduit. For mid-esophageal tumors, a bronchoscopy is also indicated.
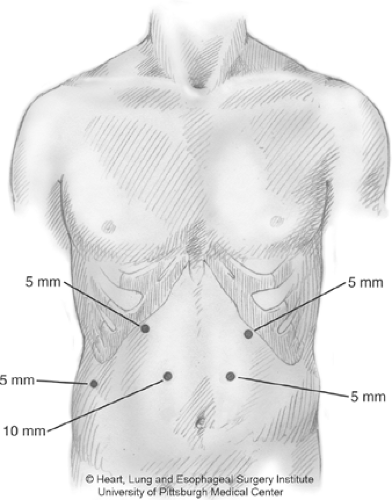
The patient is placed supine. The double-lumen endotracheal tube is placed. Five ports are utilized for the gastric mobilization. A 10 to 12 mm port is placed right of midline in the epigastrium, below the midpoint between the xiphoid process and the umbilicus. The port is inserted under direct vision using a blunt port cut-down approach. The patient is placed in steep reverse Trendelenburg position. A 5-mm port (or a 10-mm port) is placed to the left of midline at the same level as the original port. A 5-mm (or 10-mm), 30-degree laparoscopic camera is placed through this port. Additional 5-mm ports are placed at the left subcostal margin and the right subcostal margin (Fig. 1). A 5-mm port is placed in the right flank to support a liver retractor. A self-retaining retractor is used to elevate the left lobe of the liver and expose the hiatus. The gastrohepatic ligament is divided to expose the right crus. The gastroesophageal junction is freed from the hiatus by dissection up the right crus. The phrenoesophageal ligament is taken down, and the dissection is extended
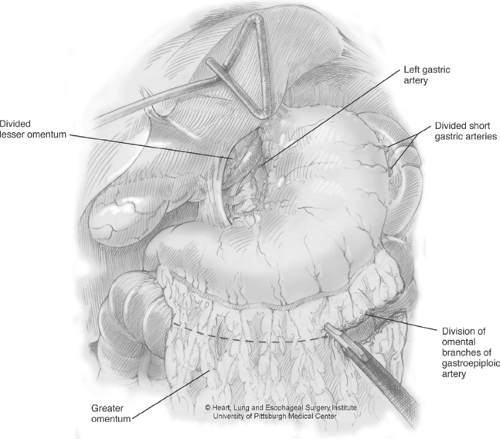
to the left crus. The right gastroepiploic arcade is identified, and the gastrocolic ligament is divided lateral to this arcade. Dissection is carried up along the greater curvature of the stomach, taking down the short gastric arteries (Fig. 2). As dissection nears the short gastric arteries, a tongue of omentum is created that extends laterally. This will be used later in the chest to buttress the anastomosis. We currently use this omental flap for all cases where preoperative radiation has been administered. Once dissection is carried up toward the left crus, the posterior attachments of the gastroesophageal junction can be divided. The stomach is retracted superiorly and to the right to expose the celiac vessels. Celiac and gastric nodal tissue is dissected free and left with the specimen. The left gastric artery is then isolated and divided at the base using an Endo-GIA vascular stapler. The stomach itself must be handled with care at all times to minimize traumatic injuries to the tissue.
to the left crus. The right gastroepiploic arcade is identified, and the gastrocolic ligament is divided lateral to this arcade. Dissection is carried up along the greater curvature of the stomach, taking down the short gastric arteries (Fig. 2). As dissection nears the short gastric arteries, a tongue of omentum is created that extends laterally. This will be used later in the chest to buttress the anastomosis. We currently use this omental flap for all cases where preoperative radiation has been administered. Once dissection is carried up toward the left crus, the posterior attachments of the gastroesophageal junction can be divided. The stomach is retracted superiorly and to the right to expose the celiac vessels. Celiac and gastric nodal tissue is dissected free and left with the specimen. The left gastric artery is then isolated and divided at the base using an Endo-GIA vascular stapler. The stomach itself must be handled with care at all times to minimize traumatic injuries to the tissue.
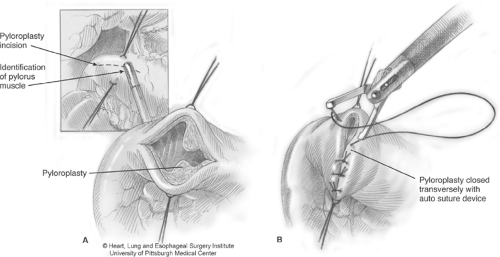
Having mobilized the stomach, a pyloroplasty is then performed in a Heineke-Mikulicz fashion (Fig. 3). Endostitches are placed superiorly and inferiorly on the pylorus to provide retraction. Ultrasonic shears are used to incise the pylorus, and the opening is closed transversely using 2-0 interrupted endosutures. A Kocher maneuver is performed, and the retrogastric and duodenal attachments are carefully dissected to achieve adequate mobilization of the gastric tube. Adequate mobilization should allow the pylorus to easily reach the right crus. This should be assessed at several time points during the mobilization, to allow the surgeon to understand the degree of dissection required. If there is any difficulty with this maneuver, further pyloroantral mobilization is generally required.
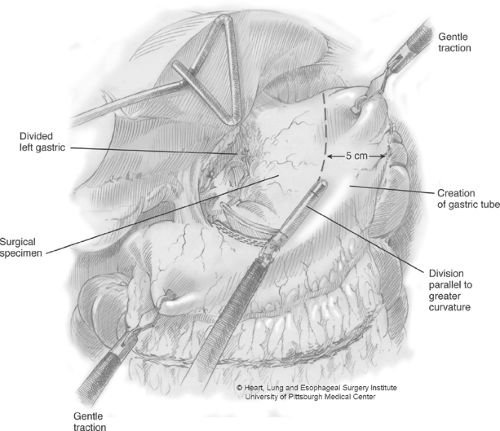
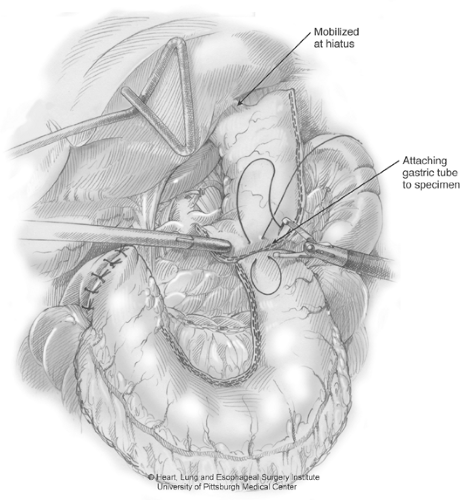
Gastric tube construction is now initiated by firing the Endo-GIA stapler across the lesser-curve vessels and fat at an angle toward the incisura. For the first firing, we generally use a vascular load, with a staple height of 2.5 mm, to minimize small-vessel oozing along the lesser curve (Fig. 4). Many times, the first firing divides mostly fatty tissue surrounding the lesser curve vessels. The right gastric vessels are preserved. The angle of the first few staple firings will be determined by the desired gastric tube diameter and should be placed accordingly. We prefer to create a gastric tube that is 4–5 cm wide. During this stapling, we apply slight caudal and simultaneous cephalad traction to keep the gastric tube on slight stretch. This affords a more consistent stapler application and a better length of the final tube. Subsequent firings of the stapler should be maintained in a line parallel to the greater-curvature arcade to create a consistent tube width and avoid spiraling of the tubularized gastric conduit. The staple load used along the thick gastric antrum requires the green stapling cartridge (4.8-mm height). As the stapling continues toward the fundus, the gastric wall thickness generally diminishes and we switch to blue loads (3.5 mm). The staple line is inspected for hemostasis. Two endosutures are used to attach the resected specimen to the gastric tube (Fig. 5). These sutures should be placed from the tip of the fundic portion of the tube to the lesser-curve part of the resected specimen. This tends to minimize the bulk as the specimen and gastric tube are passed through the hiatus.
Feeding Jejunostomy
An additional 10-mm port is placed in the right lower quadrant to facilitate jejunostomy tube placement. The transverse colon is retracted cephalad, using a grasper applied to the adjacent fatty epiploicae, and the ligament of Treitz is identified. Approximately 40 cm from the ligament of Treitz, a loop of jejunum is attached to the anterior abdominal wall in the left lower quadrant using an Endostitch. A 5-Fr needle catheter feeding jejunostomy tube is inserted into the jejunum percutaneously using a Seldinger technique (Compat Biosystems, Minneapolis, MN). Recently, Compat Biosystems stopped producing this catheter and we have been utilizing several new laparoscopic J-tube kits. The kits still employ a guidewire Seldinger technique and this guidewire is threaded into the small bowel followed by the dilator, and then the catheter to a distance of approximately 20 cm (Fig. 6). The jejunum is further tacked to the anterior abdominal wall using three additional endosutures as well as a single suture approximately 3 cm distal to the entrance site, to prevent torsion. The feeding catheter is secured on the skin, and 10 ml of air is injected rapidly into the small bowel to test for patency and confirm intraluminal placement. If any doubt exists as to true luminal placement, on-the-table intestinal contrast imaging of the J-tube should be performed (such as with diatrizoate meglumine and diatrizoate sodium (gastrograffin)).
Thoracoscopy
The patient is placed in the left lateral decubitus position. Once the position of the double-lumen tube is confirmed, the right lung should be isolated immediately, to provide adequate time for decompression. The position of the right scapula is marked. The operating table is flexed to move the iliac crest away from the costal margin and expand the intercostal spaces.
Four ports are used to access the right chest. A 10-mm camera port is inserted in the anterior axillary line at the eighth interspace. We prefer this port to be low on the chest and anterior, so that the access enters the chest just above the costophrenic recess. This affords a nice panoramic view of the chest and also, if any blood drips down the port, the blood tends to not run onto
the thoracoscope. An additional 10-mm port is placed approximately 2 cm posterior to the posterior axillary line in the eighth or ninth interspace. This is the main dissection port for the ultrasonic coagulation shears (U.S. Surgical, Norwalk, CT). A 10-mm port is placed in the fourth interspace along the anterior axillary line. A fan retractor is placed through this port to provide retraction of the lung. Finally, a 5-mm port is placed below the scapular tip. In addition, an Endostitch is placed in the central tendon of the right diaphragm and brought out percutaneously through the low anterior chest wall near the costophrenic recess using an Endo-Close device (U.S. Surgical). Downward traction on this stitch pulls the diaphragm inferiorly and allows better visualization of the lower esophagus and hiatus.
the thoracoscope. An additional 10-mm port is placed approximately 2 cm posterior to the posterior axillary line in the eighth or ninth interspace. This is the main dissection port for the ultrasonic coagulation shears (U.S. Surgical, Norwalk, CT). A 10-mm port is placed in the fourth interspace along the anterior axillary line. A fan retractor is placed through this port to provide retraction of the lung. Finally, a 5-mm port is placed below the scapular tip. In addition, an Endostitch is placed in the central tendon of the right diaphragm and brought out percutaneously through the low anterior chest wall near the costophrenic recess using an Endo-Close device (U.S. Surgical). Downward traction on this stitch pulls the diaphragm inferiorly and allows better visualization of the lower esophagus and hiatus.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree