Thus 14.3 mL of an isotonic solution of atropine sulfate contain 1 g of atropine sulfate while 14.3 mL of an isotonic solution of sodium chloride contain 0.13 g of NaCl. Therefore, 0.13 g of NaCl generates the same osmotic pressure as 1 g of atropine sulfate. In terms of osmotic pressure, each gram of atropine sulfate can be replaced by 0.13 g of NaCl. The number 0.13 is called the sodium chloride equivalent of atropine sulfate.
7.7 mL of a solution containing 1 g of streptomycin sulfate is isotonic with tears. Calculate the sodium chloride equivalent of streptomycin sulfate.

Solution. 0.069
CALCULATION
For isotonic sodium chloride solution,
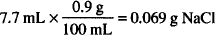
Therefore, 0.069 g of NaCl is osmotically equivalent to 1 g of streptomycin sulfate; 0.069 is the sodium chloride equivalent of this drug.


Solution. 0.18
CALCULATION
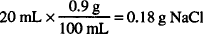
0.18 g NaCl is osmotically equivalent to 1 g penicillin G potassium.
0.18 is the sodium chloride equivalent of penicillin G potassium.
ISOTONICITY BY SODIUM CHLORIDE EQUIVALENT METHOD
If no atropine sulfate were present, the amount of sodium chloride needed for isotonicity would be
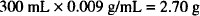
However, the solution does contain
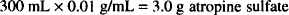
This amount of atropine sulfate exerts a certain osmotic pressure. Using the sodium chloride equivalent (0.13), we can determine the amount of sodium chloride that has the same osmotic pressure as 3.0 g of atropine sulfate:
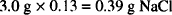
The atropine sulfate in the solution exerts an osmotic pressure equal to that of 0.39 g of NaCl. Therefore, to find the amount of sodium chloride to add, it is necessary to subtract 0.39 g from the amount of NaCl that would have been needed had the salt been the only solute in the system
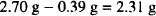
To prepare the solution, dissolve 3 g of atropine sulfate and 2.31 g of sodium chloride in sufficient water to make a total volume of 300 mL.
How many milligrams of sodium chloride should be used to prepare the following prescription?
 | Ephedrine sulfate | 0.5 g |
| NaCl | q.s. | |
| Purified water ad | 50 mL | |
| Make isotonic solution. | ||
| Sig. Eye drops |

Solution. 335 mg
CALCULATIONS
If no ephedrine sulfate were present, the amount of sodium chloride needed for isotonicity would be
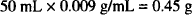
The solution contains 0.5 g ephedrine sulfate, which has a sodium chloride equivalent (E value) of 0.23.
The amount of sodium chloride that has the same osmotic pressure as 0.5 g of ephedrine sulfate is:
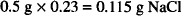
The ephedrine sulfate in die solution exerts an osmotic pressure equal to that of 0.115 g of NaCl. Therefore, to find the amount of sodium chloride to add, it is necessary to subtract 0.115 g from the amount of NaCl that would have been needed had the salt been the only solute in the system.
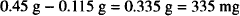
To prepare the solution, dissolve 500 mg of ephedrine sulfate and 335 mg of sodium chloride in sufficient water to make a total volume of 50 mL.

How many grams of sodium chloride should be used to make 90 mL of a 0.5% pilocarpine hydrochloride solution isotonic? The sodium chloride equivalent for pilocarpine hydrochloride is 0.24.

Solution. 0.702 g
90 mL × 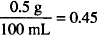 g pilocarpine hydrochloride
g pilocarpine hydrochloride
Step 1:  = 0.81 g NaCl
= 0.81 g NaCl
Step 2: 0.45 g × 0.24 = 0.108 g Nacl
Step 3: 0.81 g − 0.108 g = 0.702 g NaCl

How many grams of sodium chloride should be used to make this solution isotonic? How would you prepare this solution? (The sodium chloride equivalent (E) of phenylephrine hydrochloride is 0.32; that of zinc sulfate is 0.15.)

Solution. 0.447 g
To prepare the solution, dissolve 0.15 g of phenylephrine hydrochloride, 0.3 g of zinc sulfate, and 0.447 g of sodium chloride in sufficient water to make 60 mL.
CALCULATIONS
If NaCl were the only solute, the amount needed would be
60.0 mL ×  = 0.54 g
= 0.54 g
For phenylephrine HCl:
60 mL × 0.0025 g/mL = 0.15 g
0.15 g × 0.32 = 0.048 g NaCl
For zinc sulfate:
60 mL × 0.005 g/mL = 0.3 g
0.3 g × 0.15 = 0.045 g NaCl
The total amount of sodium chloride equivalent to the osmotic pressure exerted by both drugs is: 0.048 g + 0.045 g = 0.093 g NaCl
The amount of sodium chloride needed is: 0.54 g − 0.093 g = 0.447 g


Solution. 8.34 g
CALCULATIONS
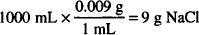
Gentamicin sulfate: 1000 mL  × 0.05 = 0.5 g
× 0.05 = 0.5 g
Benzalkonium chloride: 1000 mL × 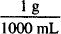 × 0.16 = 0.16 g
× 0.16 = 0.16 g
9 g − (0.5 + 0.16) g = 8.34 g NacL needed

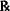 | Procaine HCl, 2% |
| Ft. 30 mL | |
| M. Ft. isotonic solution with sodium chloride. |
Explain how the formula will be compounded.

Solution.
Weigh 0.6 g of procaine HCl
Weigh 0.144 g of NaCl
Dissolve in enough water to make 30 mL of isotonic solution
CALCULATIONS
30 mL × 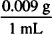 = 0.27 g of NaCl in 30 mL of an isotonic NaCl solution
= 0.27 g of NaCl in 30 mL of an isotonic NaCl solution
30 mL × 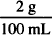 = 0.6 g of procaine HCl required
= 0.6 g of procaine HCl required
0.6 g × 0.21 = 0.126 g of NaCl represented by procaine HCl
0.27 − 0.126 = 0.144 g of NaCl required to make the solution isotonic
ISOTONIC BUFFERED SOLUTIONS
The USP describes two methods but we will focus on the White–Vincent method.
The White–Vincent method is based on the following equation:
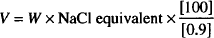
where, W is the weight of the drug (in grams) and V is the volume (in mL) of isotonic solution that can be prepared with W grams of drug.
As an example, we will prepare an isotonic solution and make it to a final volume with an isotonic vehicle.
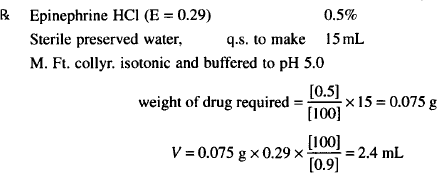
Conclusion: Dissolve 0.075 g epinephrine HCl in sterile H2O up to 2.4 mL and complete the volume to 15 mL with an isotonic vehicle buffered to pH 5.0.
Use the White–Vincent method in the following example.



Solution. Dissolve 0.6 g ingredient Z in water up to 12 mL and complete the volume to 30 mL with isotonic acetate buffer.
CALCULATIONS
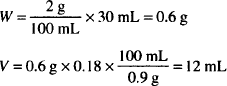

 | Homatropine hydrobromide (E = 0.17) | 1% |
| M. Ft. collyr. isotonic | 60 mL |
The USP recommends ophthalmic solutions of salts of homatropine to be buffered at pH 6.8 and suggests a formula for a suitable isotonic phosphate buffer.

Solution. Dissolve 0.6 g homatropine HBr in sterile H2O up to 11.3 mL and dilute to 60 mL with isotonic phosphate buffer pH 6.8.
CALCULATIONS
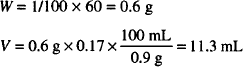
Then, the prescription would be filled as follows:
| Homatropine hydrobromide | 600 mg | |
| Sterile water, | to make | 11.3 mL |
| Isotonic phosphate buffer, pH 6.8 q.s. | ad | 60 mL |

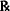 | Tetracaine hydrochloride (E = 0.18) | 0.5% |
| M. Ft. collyr. isotonic | 30 mL |

Solution. Dissolve 0.15 g tetracaine HCl in sterile H2O up to 3 mL and dilute to 30 mL with boric acid vehicle.
CALCULATIONS
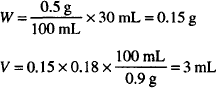

 | Oxytetracycline hydrochloride (E = 0.12) | 0.05 g |
| M. Ft. collyr. Isotonic | 30 mL |
Use boric acid vehicle to make to final volume.

Solution. Dissolve 50 mg oxytetracycline HCl in sterile H2O up to 0.7 mL and dilute to 30 mL with boric acid vehicle.
CALCULATIONS
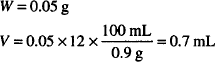
OTHER TONICITY AGENTS
If a substance other than sodium chloride is to be employed to raise osmotic pressure, the calculation procedure requires one extra step.
To begin, follow the same procedure as though sodium chloride were to be used.
Extra step: After calculating the amount of sodium chloride needed, divide that amount by the sodium chloride equivalent of the substance (tonicity agent) that will be used to raise the osmotic pressure of the solution. This produces the required result.
As an example, let us calculate the amount of sodium nitrate needed to make 200 mL of a 0.6% solution of silver nitrate isotonic. The sodium chloride equivalent of silver nitrate is 0.33. That of sodium nitrate is 0.68.
If only sodium chloride were present, the amount needed would be
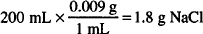
The amount of silver nitrate needed is
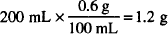
This is osmotically equivalent to
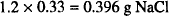
The amount of sodium chloride that would have to be added is
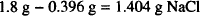
To find the amount of sodium nitrate that should be used to adjust osmotic pressure, we divide by the sodium chloride equivalent of that substance.
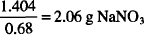
Calculate the amount of boric acid that should be included in 300 mL of a 0.4% procaine hydrochloride solution to render the solution isotonic. Procaine hydrochloride, E = 0.21; boric acid, E-value = 0.52.

Solution. 4.7 g
CALCULATIONS


 | Ephedrine sulfate (E = 0.23) | 0.3 g |
| Chlorobutanol (E = 0.24) | 0.15 g | |
| Dextrose monohydrate (E = 0.16) | q.s. | |
| Rose water ad | 30 mL | |
| Make solution isotonic with nasal fluid. |
Calculate the quantity of dextrose monohydrate to use

Solution. 1.03 g
CALCULATIONS
30 mL ×  = 0.27 g NaCl
= 0.27 g NaCl
0.3 g × 0.23 = 0.069 g NaCl represented by ephedrine sulfate
0.15 g × 0.24 = 0.036 g NaCl represented by chlorobutanol
0.27 g − (0.069 + 0.036) = 0.165 g NaCl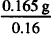 = 1.03 g dextrose
= 1.03 g dextrose

 | Tetracaine hydrochloride Sol. 2% | 15 mL |
| Epinephrine bitartrate (E = 0.18) | 0.1% | |
| Boric acid (E = 0.52) | q.s. | |
| Sterile water ad | 30 mL | |
| M. Ft. sol. isot. with tears. |
The solution of tetracaine HCl 2% is already isotonic. How many milliliters of a 2.5% solution of boric acid should be used in compounding the prescription?

Solution. 10 mL of 2.5% boric acid solution
CALCULATIONS
30 mL (total volume prescribed) − 15 mL (already isotonic) = 15 mL (to be made isotonic)
15 mL ×  = 0.135 g NaCl (total needed)
= 0.135 g NaCl (total needed)
Because the amount of drug will be present in the total volume,
30 mL ×  = 0.03 g epinephrine bitartrate
= 0.03 g epinephrine bitartrate
0.03 g × 0.18 = 0.0054 g NaCl represented by ephedrine
0.135 − 0.0054 = 0.1296 g NaCl (to be added)
 boric acid
boric acid
 mL boric acid solution 2.5%
mL boric acid solution 2.5%
Conclusion: Dissolve 0.03 g epinephrine bitartrate in 10 mL of 2.5% boric acid solution and make the volume to 15 mL with sterile water. Add 15 mL isotonic tetracaine hydrochloride sol. 2% to obtain 30 mL prescription.
Now it’s your turn:
 | Oxytetracycline HCl(E = 0.12) | 2% |
| Chlorobutanol sol. 0.01% | 10 mL | |
| Sodium chloride | q.s. | |
| Sterile water ad | 30 mL | |
| Make solution isotonic with tears. |
The 0.01% solution of chlorobutanol is already isotonic. How many milliliters of a 0.9% solution of NaCl should be used to prepare the prescription? Explain how you would prepare the solution.

Solution. Use 12 mL of 0.9% NaCl solution. Dissolve 0.6 g oxytetracycline HCl in 12 mL NaCl 0.9% and make volume to 20 mL with sterile water. Add 10 mL chlorobutanol sol. 0.01% to obtain 30 mL prescription.
CALCULATIONS
30 mL − 10 mL = 20 mL

0.6 g × 0.12 = 0.072 g NaCl
0.18 − 0.072 = 0.108 g NaCl
0.108 g ×  g = 12 mL NaCl 0.9%
g = 12 mL NaCl 0.9%

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


