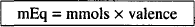Let us go through a couple of problems as a review.
The chemical equation for the dissociation of calcium nitrate (Ca (NO3)2) is:
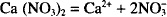
Thus, one mol of calcium nitrate yields 1 mol of calcium ion and 2 mols of nitrate ion. How many mols result from the dissociation of 1 mol of sodium sulfate? (Na2SO4)
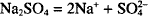
Each mol of sodium sulfate yields 2 mols of sodium and 1 mol of sulfate.
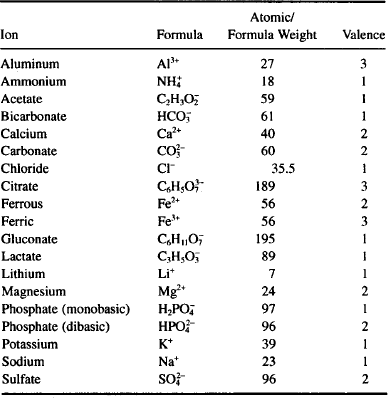
Now it’s your turn to practice, using elementary inorganic chemistry skills and data provided in Table 9.1 and Appendix 4 (names, symbols, atomic weight of some elements with pharmaceutical importance).
TABLE 9.1 Properties of some important ions
How many mols of magnesium chloride (MgCl2) are necessary to yield 1 mol of magnesium ion?

Solution. MgCl2 = Mg2++ 2Cl−
Each mol of magnesium chloride yields 1 mol of magnesium ion.


Solution. One-half mol of MgCl2
MgCl2 = Mg2+ + 2Cl−
Each mol of magnesium chloride yields 2 mols of chloride ion.

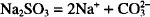
Each mol of sodium carbonate contains 2 mols of sodium. The molecular weight of sodium carbonate is 106. Therefore, 106 g of sodium carbonate contain 46.0 g of sodium.
How many grams of anhydrous sodium carbonate contain 350 mg of sodium?

Solution. 0.807 g
CALCULATIONS
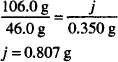


Solution. 40%
Calcium has an atomic weight of 40.0.
CaCO3 has a molecular weight of 100.0.
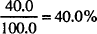
MILLIMOL (MMOL)
If you feel you need a review, check these examples and try the next two problems. Otherwise, go to frame 8.
How many milligrams of sodium chloride represent 0.5 mmol?
Molecular wt of NaCl = 58.5
58.5 mg NaCl = 1 mmol
0.5 mmol = 29.3 mg
How many millimols are there in 3.2 g of calcium fluoride?
MW of CaF2 = 78.0
78.0 mg CaF2 = 1 mmol
3200 mg × 1 mmol/78.0 mg = 41 mmol
A prescription calls for 24 mmol of potassium chloride. How many grams of KCl are required?

Solution. 1.79 g
CALCULATIONS
MW of KCl = 74.5 and 1788 mg = 1.79 g
74.6 mg = 1 mmol
24 mmol 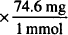 = 1790 mg = 1.79 g
= 1790 mg = 1.79 g

 | NaCl solution 90.0 mL |
| Each 5 mL should contain 0.6 mmol of Na+. |

Solution. 0.632 g
CALCULATIONS
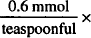 18 teaspoonfuls = 10.8 mmol Na+
18 teaspoonfuls = 10.8 mmol Na+
NaCl = Na+ + Cl−
From this equation we see that each mol of sodium chloride yields 1 mol of Na+.
Therefore, 10.8 mmol of Na+ will be supplied by 10.8 mmol of NaCl. Thus,
MW of NaCl = 58.5
58.5 mg NaCl = 1 mmol
10.8 mmol 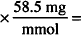 632 mg = 0.632 g
632 mg = 0.632 g
EQUIVALENT AND MILLIEQUIVALENT (mEq)
Since total positive and negative charges in a salt must balance, we can define the valence of a salt as the sum of either the positive or negative charges. For example, the valence of CaCl2, which contains two positive charges and two negative charges, is 2.
Write the valence of each of the following compounds:

Solutions.


Solution. 2 equivalents of each ion.
K2CO3 = 2K+ + CO32−
Thus 2 mol of K+ are produced
mols × valence = equivalents
2 × 1 = 2 equivalents of K+
Only 1 mol of carbonate is produced.
mols × valence = equivalents
1 × 2 = 2 equivalents of CO32−
Notice that the equivalents of cation and anion are equal in number.

Since the number of millimols of a drug in solution may be calculated by dividing the amount of drug, in mg, by its molecular weight, then the following may also be used:
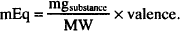
In addition, if we rearrange its terms, the number of milligrams of drug can be determined if a given number of milliequivalents of drug are known:
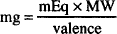
Now, check the following example solved by each approach.
How many mg/mL are present in a solution containing 2 mEq/mL of KCl? (MW = 74.5)
Solving by (a):
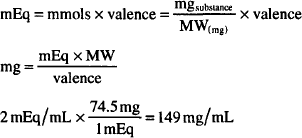
or

Solving by (b):
MW of KCl = 74.5 g
Equiv. weight of KCl = 74.5/1 = 74.5 g (valence = 1)
1 mEq of KCl = 1/1000 × 74.5 g = 0.0745 g = 74.5 mg
2 mEq = 2 × 74.5 mg = 149 mg/mL
Solving by (c):
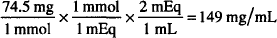


Solution. 16.7 mEq
CALCULATIONS
MW of MgSO4 = 120
EqW = 120/2 = 60 g
1 mEq = 60 mg
1 g = 1000 mg
1000 mg × 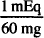 = 16.7 mEq
= 16.7 mEq
or
MW of MgSO4 = 120
1 mmol MgSO4 = 120 mg
EqW = 120 g/2 = 60 g
1 mEq = 1/1000 × 60 g = 0.060 g = 60 mg
1 mmol = 2 mEq (120 = 2 × 60)
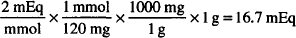


Solution. 3 mg%
CALCULATIONS
Atomic weight of magnesium = 24 g
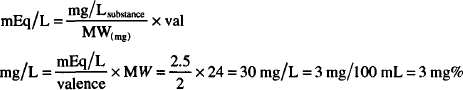
or
Atomic weight of magnesium = 24 g
Eq. weight of Mg2+ = 24/2 = 12 g
1 mEq = 12 mg
2.5 mEq/L = 2.5 × 12 = 30 mg/L = 3 mg/100 mL = 3 mg%


Solution. 780 mL
CALCULATIONS
MW of NaCl = 58.5 g
EqW = 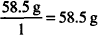
1 mEq = 0.0585 g
2 mEq/kg dose = 2 × 0.0585 = 0.117 g/kg
132 lb = 60 kg (1 kg = 2.2 lb)
0.117 g × 60 = 7.02 g of NaCl needed
0.9% NaCl available = 0.9 g/100 ml
7.02 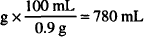
or
132 lb 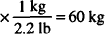
60 kg × 2 mEq/kg = 120 mEq needed
mg = 



Solution. 6.02 mEq
CALCULATIONS
KF = K+ + F−
1 mEq F− = 1 mmol F− = 1 mmol KF = 58.1 mg
350 mg KF × 1 mEq F−/58.1 mg KF = 6.02 mEq F−
or
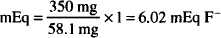


Solution. 2.10 mEq
CALCULATIONS
(5 mL × 0.02 g/mL) = 0.1 g MgCl2 = 100 mg
MgCl2 = Mg2+ + 2 Cl−
1 mmol MgCl2 = 95.3 mg = 1 mmol Mg2+ = 2 mEq Mg2+
100 mg MgCl2 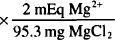 = 2.10 mEq Mg2+
= 2.10 mEq Mg2+
or



Solution. 6.62 g
CALCULATIONS
2.5 mEq/teaspoonful × 36 teaspoonfuls = 90 mEq needed
CaCl2 · 2H2O = Ca2+ + 2Cl− + 2H2O
1 mmol Ca2+ = 2 mEq Ca2+ = 1 mmol CaCl2 · 2H2O = 147.0 mgCaCl2 · 2H2O
90 mEq 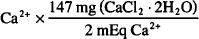 = 6620 mg = 6.62 g CaCl2 · 2 H2O
= 6620 mg = 6.62 g CaCl2 · 2 H2O

 | Solution calcium chloride 5% | 180 mL |
| Sig: 1 teaspoonful tid |
 | Potassium sulfate to yield 1.0 mEq of K+ | |
| Aqua qs ad | 5.0 mL | |
| d.t.d. #24 | ||
| Sig: 5.0 mL b.i.d. |

Solutions.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree