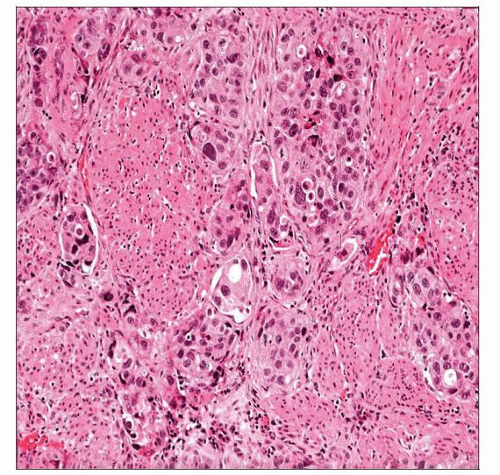
Invasive Urothelial Carcinoma
Jesse K. McKenney, MD
Key Facts
Terminology
Urothelial carcinoma that invades beyond basement membrane
Clinical Issues
Invasion into lamina propria usually managed with intravesical therapy
Invasion into muscularis propria usually managed by radical cystectomy
Microscopic Pathology
Small nests, clusters, &/or single cells within lamina propria
Surrounding retraction artifact is common
Other stromal reactions include desmoplasia, sclerosis, and myxoid change
May have irregular, jagged tongues of epithelium in continuity with overlying noninvasive component
Top Differential Diagnoses
Prostatic adenocarcinoma involving bladder
Gynecologic carcinomas involving bladder
Paraganglioma
Inverted patterns of noninvasive urothelial neoplasia
Nephrogenic adenoma
Pseudocarcinomatous hyperplasia
Diagnostic Checklist
Important to state depth of invasion by clearly reporting invasion of “lamina propria” or “muscularis propria”
Recognizing heterogeneity of urinary bladder microanatomy is important to avoid overstaging
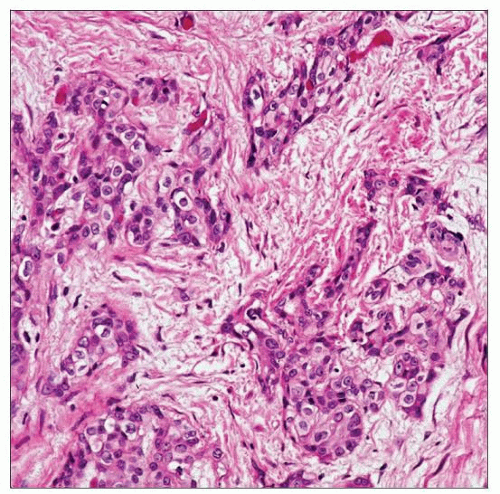 The irregular, jagged nests of urothelium present in this example of urothelial carcinoma are diagnostic of stromal invasion into the lamina propria. No muscularis propria is seen. |
TERMINOLOGY
Synonyms
Invasive transitional cell carcinoma
Definitions
Urothelial carcinoma that invades beyond basement membrane
CLINICAL ISSUES
Treatment
Surgical approaches
Transurethral resection of visible tumor to base
Required for accurate assessment of invasion
Invasion into lamina propria usually managed conservatively with intravesical therapy
Bacillus Calmette-Guérin
Mitomycin and other intravesical therapies
Radical cystectomy rarely performed, institution dependent
Invasion into muscularis propria usually managed by radical cystectomy
± neoadjuvant therapy
Radiation therapy is primary treatment modality in some cases
Prognosis
Stage dependent
Deeply invasive tumors (pT2 or greater/muscularis propria and beyond)
Poor prognosis
Superficially invasive tumors (pT1/lamina propria)
May have excellent prognosis
MACROSCOPIC FEATURES
General Features
May be papillary, polypoid, nodular, solid, or ulcerated
Background urothelium may be normal or erythematous
Frequently multifocal
MICROSCOPIC PATHOLOGY
Key Descriptors
Predominant Cell/Compartment Type
Epithelial, urothelial
Normal Histologic Anatomy of Bladder Wall
Detailed knowledge of bladder microanatomy is required for proper pathologic staging
Lamina propria
Hypocellular collagenized or edematous stroma
Stromal cells may be hyperchromatic and multilobated
Associated small to medium caliber blood vessels
Muscularis mucosae
Classically has thin, wispy fascicles of smooth muscle
When hyperplastic, fascicles may be thicker and disorganized with dispersion in multiple directions
Occasionally, individual small rounded thick muscle bundles separated by stroma are present in lamina propria
May contain adipose tissue
Muscularis propria (detrusor muscle)
Large aggregates of confluent dense smooth muscle
Often contains adipose tissue
May be very superficially located in some regions
Patterns of Invasion
Small nests or clusters/single cells within lamina propria
Surrounding retraction artifact is common
Other stromal reactions include desmoplasia, sclerosis, and myxoid change
Microinvasion: Focal invasion of single cells or small clusters, < 2 mm in depth
May have more abundant eosinophilic cytoplasm than adjacent noninvasive component
“Paradoxical maturation”
May have irregular, jagged tongues of epithelium in continuity with overlying noninvasive component
Most invasive urothelial carcinomas are high grade
Exceptions are nested and tubular variants
Grade of invasive component does not affect prognosis as all have recurring and metastatic potential
ANCILLARY TESTS
Immunohistochemistry
Usually immunoreactive for p63, CK20, and HMCK(34βE12)
Low specificity
Uroplakin, thrombomodulin, GATA3, and S100p are more specific markers of urothelial lineage
Relatively low sensitivity
Smoothelin immunostains may be helpful in distinguishing muscularis mucosae from muscularis propria
Weak, patchy staining in muscularis mucosae
Strong, diffuse reactivity in muscularis propria
May be useful when tumor obliterates muscularis propria and only scant residual muscle is seen
Cytokeratin stains may be useful in identifying subtle foci of invasive carcinoma
Should not be confused with cytokeratin positive myofibroblasts
Spindled cells with tapered cytoplasmic processes
Also coexpress actin-sm
DIFFERENTIAL DIAGNOSIS
Other Nonurothelial Neoplasms
Prostatic adenocarcinoma involving bladder
Monomorphic round cells with prominent nucleoli
May have gland/acinar formation
Immunoreactive for PSA &/or PAP
Usually negative for p63 and HMCK(34βE12)
CK7/CK20 immunophenotype is highly variable in high-grade prostatic adenocarcinomas
Gynecologic carcinomas involving bladder
Cervical squamous cell carcinomas may mimic urothelial carcinoma with squamous differentiation
High-grade uterine carcinomas may mimic poorly differentiated urothelial carcinoma or urothelial carcinoma with glandular differentiation
Often express ER &/or WT1
Clinical/radiographic correlation is critical
Paraganglioma
Nested aggregates of epithelioid cells
Often have closely associated surrounding vascular network
Sclerotic/hyalinized examples may be pseudoinfiltrative
Closely mimics invasive carcinoma
May have scattered pleomorphic cells
“Endocrine anaplasia”
Immunophenotype is distinctive
Positive for synaptophysin but not cytokeratins
S100(+) sustentacular cells may be seen
Inverted patterns of noninvasive urothelial neoplasia
Crowded endophytic nests or trabeculae of urothelium with sharp rounded contours
Range from inverted papilloma to inverted high-grade carcinoma, based on cytologic features
No surrounding retraction or other stromal changes
No jagged nests
Benign Mimics
Nephrogenic adenoma
Small papillae lined by single cuboidal epithelial layer
Small tubules in lamina propria
Tubules often possess thick basement membrane
Lined by flattened or “hobnail” cells
Small lumina may resemble blood vessels
Rare “diffuse” or solid pattern may closely mimic malignancy
Diffuse nuclear pax-2/pax-8 immunoreactivity
May also stain with AMACR (P504s)
Pseudocarcinomatous hyperplasia
Often associated with prior radiation or chemotherapy
Rare cases have no prior therapy
Often have factors predisposing to ischemia
“Squamoid” epithelial nests in lamina propria may be jagged but are associated with fibrin and blood vessels
Epithelial aggregates characteristically envelop blood vessels that are obliterated by fibrin
Lamina propria is often hemorrhagic with extravasated fibrin
Other radiation-associated changes may be seen
Cystitis cystica/glandularis
Invaginated urothelial nests with superficial location in lamina propria
Rounded contours of nests
May have lobular architecture
Sharp border with lamina propria at base
No stromal reaction
Intestinal type may have associated mucin extravasation
May closely mimic malignancy clinically
DIAGNOSTIC CHECKLIST
Clinically Relevant Pathologic Features
“Hypertrophied” patterns of muscularis mucosa are not restricted to men with prostatic hyperplasia
Pathologic Interpretation Pearls
Recognizing heterogeneity of urinary bladder microanatomy is important to avoid overstating
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree