Invasive Micropapillary Carcinoma
ADRIANA D. CORBEN
EDI BROGI
Invasive micropapillary carcinoma is a morphologically distinctive form of mammary carcinoma in which tumor cells are arranged in morule-like clusters devoid of fibrovascular cores and situated within empty stromal spaces. Fisher et al.1 referred to this configuration as an “exfoliative appearance.” This growth pattern may be found throughout the lesion (pure invasive micropapillary carcinoma) or as part of an otherwise conventional invasive duct carcinoma (mixed invasive micropapillary carcinoma). Criteria for distinguishing between mixed and “pure” invasive micropapillary carcinoma remain imprecise. Some authors have used the term “micropapillary” for lesions with micropapillary growth in at least 50% of the tumor. Others restrict the term to tumors that consist entirely of this pattern, an exceedingly rare occurrence. In practical terms, to be considered in the “pure” category, at least 75% of the invasive tumor should be micropapillary. Some mucin-producing carcinomas can also have this growth pattern, but there is no consensus on whether these tumors are variants of invasive micropapillary or mucinous carcinomas.
CLINICAL PRESENTATION
Age and Gender
The reported age at diagnosis ranges from 25 to 89 years.2,3,4,5,6,7,8,9 The median ages in several studies were 46,8 48.9,9 50,6 57,2,10 and 624 years. The mean ages in four series were 50,5 52.3,11 53.5,7 and 5812 years. Luna-Moré et al.13 observed that patients whose tumors contained more than 50% micropapillary carcinoma tended to be older than patients with focal micropapillae. Rare cases of invasive micropapillary carcinoma have been described in male patients.2,9,14,15,16 No specific genetic or ethnic association has been identified.
Clinical Examination
Most patients present with a palpable breast mass, but occasional lesions are detected mammographically as a density or because of microcalcifications.4,5,7,9 One tumor clinically mimicked a hematoma because of extensive cystic degeneration and intratumoral hemorrhage.7 Yun et al.9 described nipple retraction, a hematoma, and diffuse skin thickening in one patient each. Two patients had nipple discharge.9 Presentation as an “axillary mass” was described in one patient and in the subareolar region in another three.5 The distribution of tumors in terms of laterality and location in the breast does not differ significantly from that of ordinary invasive ductal carcinomas.4,5,12,13
Radiology
The imaging features of invasive micropapillary carcinomas are highly suggestive of carcinoma. Yun et al.9 evaluated the radiologic findings in a series of 29 patients. Nineteen of 29 (65.5%) carcinomas presented as a palpable mass, 7/29 (24.1%) with a screening abnormality, 1/29 (3.4%) with nipple discharge, and 1/29 (3.4%) as a mass and nipple discharge. One tumor was detected during follow-up of prior breast surgery. A mammogram that was available for 24/29 patients showed a mass (54.2%), an area of asymmetry (20.8%), and calcifications without mass or asymmetry (20.8%). One patient had no mammographic abnormalities. Calcifications were detected in 16 cases (66.7%), and most were the fine pleomorphic type (56.3%). Their distribution was often segmental (50%) or clustered (37.5%). By ultrasound, it was found that the tumor mass had an irregular shape (25/29; 86.2%), parallel orientation (25/29; 86.2%), a spiculated margin (17/29; 58.6%), an abrupt interface with the surrounding tissue (22/29; 75.9%), and a hypoechoic pattern (27/29; 93.1%). Magnetic resonance imaging (MRI) detected a mass with enhancement in 11/18 (61.1%) cases and non-mass-like enhancement in 7/18 (38.9%) cases. Kamitani et al.17 reported the ultrasonographic findings of six invasive micropapillary carcinomas. Half of the tumors had uniform echogenicity compared with subcutaneous fat tissue. The authors speculated that the presence of a central lumen in the tumor clusters might have contributed to the relatively high internal echogenicity of these carcinomas.
GROSS PATHOLOGY
Tumor size in several reports ranged from 0.1 to 11 cm, with medians of 1.5,4 2.3,18 2.8,2 3.38,6 and 3.9 cm11 in five different series. The mean size of eight tumors in a series studied by Uddin et al.7 was 2.9 cm (1.7 to 4.5 cm). Another study described larger lesions with a mean size of 4.9 cm, with only
14.8% of T1 lesions, in contrast to 51.8% of T2, and 33.3% of T3 tumors.2 In the same series, tumors with more than 50% of micropapillary growth tended to be larger (mean, 6 cm) than those with a lesser amount of this pattern (mean, 3.5 cm). Chen et al.6 observed a similar trend in a study comparing 100 invasive carcinomas with micropapillary features and 100 invasive ductal carcinoma not otherwise specified (NOS) (3.38 vs. 2.39 cm; p < 0.001). The lesions may be multifocal grossly.2
14.8% of T1 lesions, in contrast to 51.8% of T2, and 33.3% of T3 tumors.2 In the same series, tumors with more than 50% of micropapillary growth tended to be larger (mean, 6 cm) than those with a lesser amount of this pattern (mean, 3.5 cm). Chen et al.6 observed a similar trend in a study comparing 100 invasive carcinomas with micropapillary features and 100 invasive ductal carcinoma not otherwise specified (NOS) (3.38 vs. 2.39 cm; p < 0.001). The lesions may be multifocal grossly.2
MICROSCOPIC PATHOLOGY
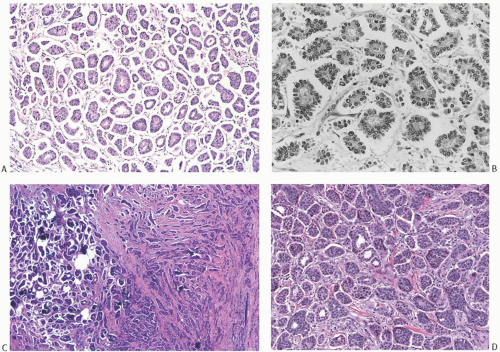
Invasive micropapillary carcinoma consists of small clusters of neoplastic epithelial cells suspended in tight clear spaces, in a growth pattern that closely mimics lymphovascular invasion (Fig. 29.1). The clusters often have a serrated outer border. They are devoid of fibrovascular cores and display an “inside-out” arrangement with the luminal aspect of the cell present on the outer surface of the cluster. A central clear space is usually present, but solid groups may also occur (Fig. 29.1). Uncommon variants feature microcystic dilation of lumens within cell clusters (Fig. 29.2) or apocrine differentiation (Fig. 29.3). The neoplastic epithelial cells are cuboidal to columnar and display finely granular or dense eosinophilic cytoplasm. Nuclear grade is usually intermediate to high,11 and mitotic activity is greater in higher grade lesions. Paterakos et al.12 reported that invasive micropapillary carcinomas had a significantly increased proportion of high-grade features and a high mitotic rate. Chen et al.6 observed that 32% of invasive carcinomas with micropapillary features were histologic grade III/III, 37% grade II/III, and 31% histologic grade I/III. Kim et al.16 compared the nuclear grade of 38 invasive micropapillary carcinomas and 217 nonmicropapillary invasive duct carcinomas and found no significant differences, but the invasive micropapillary carcinomas were significantly larger (mean tumor size 3.8 vs. 2.5 cm; p = 0.001) and more often had lymphovascular
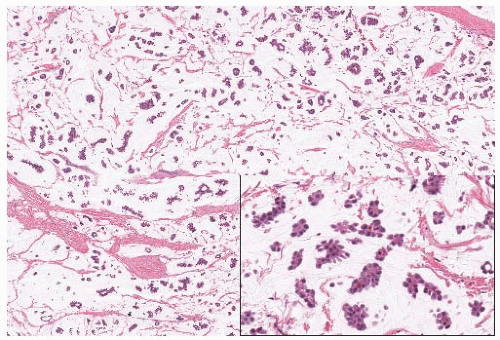
invasion (60.5% vs. 18.6%; p < 0.001). On the other hand, Yu et al.8 observed significantly higher nuclear grade in 72 invasive micropapillary carcinomas than in 144 control tumors matched for age, size, and stage (52.8% vs. 37.5%; p = 0.0387). Lymphovascular invasion (Fig. 29.4) (68.1% vs. 38.2%; p < 0.0001) and lymph node metastases with extracapsular extension (Fig. 29.4) (40.3% vs. 28.9%; p = 0.001) were also significantly more common in invasive micropapillary carcinomas than in control tumors. Intracytoplasmic mucin may be present rarely in the tumor cells. Perineural invasion occurs occasionally. Necrosis5 and a lymphocytic infiltrate4 are not typical features of invasive micropapillary carcinoma, but in some instances a lymphoid infiltrate may permeate the stroma.
invasion (60.5% vs. 18.6%; p < 0.001). On the other hand, Yu et al.8 observed significantly higher nuclear grade in 72 invasive micropapillary carcinomas than in 144 control tumors matched for age, size, and stage (52.8% vs. 37.5%; p = 0.0387). Lymphovascular invasion (Fig. 29.4) (68.1% vs. 38.2%; p < 0.0001) and lymph node metastases with extracapsular extension (Fig. 29.4) (40.3% vs. 28.9%; p = 0.001) were also significantly more common in invasive micropapillary carcinomas than in control tumors. Intracytoplasmic mucin may be present rarely in the tumor cells. Perineural invasion occurs occasionally. Necrosis5 and a lymphocytic infiltrate4 are not typical features of invasive micropapillary carcinoma, but in some instances a lymphoid infiltrate may permeate the stroma.
A clear space defined by intervening stroma consisting of dense fibrocollagenous tissue or a more delicate network of reticular tissue surrounds each tumor cell cluster. The resulting sponge-like pattern of spaces filled by tumor clusters characterizes primary tumors as well as metastatic lesions (Fig. 29.4). The clear spaces are not lined by endothelium,4 and they are usually attributed to artifactual shrinkage of stromal elements secondary to formalin fixation. Although some authors reported that clearing around the clusters is not observed in frozen sections,4 Acs et al.19 documented clear spaces around tumor clusters in the frozen section from at least one case of invasive micropapillary carcinoma. These authors suggested that the clear spaces are points of weaker stromal density that facilitate tissue infiltration by the neoplastic cells.
The spaces generally appear to be empty, but in some instances mucinous material has been demonstrated with special stains.13 Rare examples of invasive carcinoma composed
entirely of micropapillary clusters in mucin-filled spaces have been reported20,21 (Fig. 29.5). There is no agreement on whether these tumors constitute a variant of mucinous or invasive micropapillary carcinoma. Myxoid stroma has been noted in a minority of cases.4 Microcalcifications, which can be psammomatous, are variably present in the tumor cell clusters (Figs. 29.2 and 29.3).
entirely of micropapillary clusters in mucin-filled spaces have been reported20,21 (Fig. 29.5). There is no agreement on whether these tumors constitute a variant of mucinous or invasive micropapillary carcinoma. Myxoid stroma has been noted in a minority of cases.4 Microcalcifications, which can be psammomatous, are variably present in the tumor cell clusters (Figs. 29.2 and 29.3).
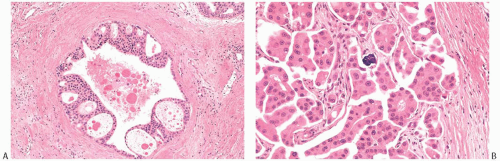 FIG. 29.3. Invasive micropapillary carcinoma, apocrine. A: Intraductal apocrine carcinoma with peripheral arcades. B: Invasive micropapillary apocrine carcinoma with a calcification. |
 FIG. 29.4. Invasive micropapillary carcinoma, lymphovascular invasion and lymph node metastases. A: Dilated lymphatic channels adjacent to invasive micropapillary carcinoma are filled with tumor emboli. Micropapillary DCIS is also present. B: The intravascular tumor emboli are morphologically indistinguishable from the micropapillary clusters invading the stroma (same tumor as in image A). C: A lymph node metastasis shows the characteristic micropapillary arrangement. D: Lymph node metastasis of micropapillary carcinoma with extracapsular extension. |
Pure invasive micropapillary carcinomas are rare. In one series,11 4.83% of 1,056 consecutive cases reviewed at one center over a 9-month period had micropapillary areas. The invasive micropapillary component represented less than 25% of the tumor mass in 9 cases (18%), 25% to 49% in 11 cases (22%), 50% to 75% in 12 cases (24%), and more than 75% in 19 cases (37%). The last group comprised 1.80% of all breast carcinomas in the study period.
Luna-Moré et al.13 found invasive micropapillary differentiation in 27 (2.7%) of 986 consecutive breast carcinomas. In 15 of the tumors, the invasive micropapillary component occupied more than 50% of the lesion. Pure invasive micropapillary carcinoma was present in 21 (1.7%) of 1,287 tumors reviewed by Paterakos et al.12 Pettinato et al.2 found an invasive micropapillary component in 62 (3.8%) of 1,635 carcinomas. The micropapillary pattern comprised 50% to 100% of 40 tumors (64.5%), 25% to 50% of 12 tumors (19.4%), and less than 25% of 10 (16.1%). About 4% of invasive ductal carcinomas studied by Kuroda et al.3 were either pure or mixed forms of invasive micropapillary carcinoma. Out of 100 carcinomas with invasive micropapillary features reported by Chen et al.,6 45 (45%) had more than 75% micropapillary morphology, 26 (26%) had micropapillae in 50% to 75% of the tumor, 15 (15%) in 25% to 49%, and 14 (14%) in less than 25%. In the same study, the type of carcinoma associated with the invasive micropapillary component was ductal NOS in 68 cases, mucinous in 2, and invasive lobular in 1 case. In the study by Yun et al.,9 the nonmicropapillary components coexisting with areas of invasive micropapillary carcinoma included conventional invasive ductal carcinoma (18 cases), invasive cribriform carcinoma (4 cases),
mucinous carcinoma (2 cases), tubular carcinoma (1 case), and microinvasive ductal carcinoma (1 case).
mucinous carcinoma (2 cases), tubular carcinoma (1 case), and microinvasive ductal carcinoma (1 case).
Mixed micropapillary carcinomas usually show a sharp demarcation between the micropapillary and NOS components (Fig. 29.1).
Ductal carcinoma in situ (DCIS) is detected in most cases.5 In pure invasive micropapillary carcinoma, the DCIS tends to be micropapillary or cribriform, with intermediate nuclear grade22 (Figs. 29.3, 29.4, and 29.6




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree