Types of ion channels and neurotransmitter receptors in the CNS: A shows a voltage-gated ion channel in which the voltage sensor controls the gating (broken arrow). B shows a ligand-gated ion channel in which binding of the neurotransmitter to the ionotropic channel receptor controls the gating. C shows a metabotropic receptor coupled to a G protein that can interact directly with an ion channel. D shows a receptor coupled to a G protein that activates an enzyme; the activated enzyme generates a diffusible second messenger, for example, cAMP, which interacts to modulatean ion channel.
(Reproduced, with permission, from Katzung BG, editor: Basic & Clinical Pharmacology, 11th ed. McGraw-Hill, 2009: Fig. 21-2.)
Types of Receptor-Channel Coupling
In the case of ligand-gated ion channels, activation (or inactivation) is initiated by the interaction between chemical neurotransmitters and their receptors (Figure 21-1). Coupling may be (1) through a receptor that acts directly on the channel protein (B), (2) through a receptor that is coupled to the ion channel through a G protein (C), or (3) through a receptor coupled to a G protein that modulates the formation of diffusible second messengers, including cyclic adenosine monophosphate (cAMP), inositol trisphosphate (IP 3), and diacylglycerol (DAG), which secondarily modulate ion channels (D).
Role of the Ion Current Carried by the Channel
Excitatory postsynaptic potentials (EPSPs) are usually generated by the opening of sodium or calcium channels. In some synapses, similar depolarizing potentials result from the closing of potassium channels. Inhibitory postsynaptic potentials (IPSPs) are usually generated by the opening of potassium or chloride channels. For example, activation of postsynaptic metabotropic receptors increases the efflux of potassium. Presynaptic inhibition can occur via a decrease in calcium influx elicited by activation of metabotropic receptors.
High-Yield Terms to Learn
Voltage-gated ion channels Transmembrane ion channels regulated by changes in membrane potential Ligand-gated ion channels Transmembrane ion channels that are regulated by interactions between neurotransmitters and their receptors (also called ionotropic receptors) Metabotropic receptors G-protein-coupled receptors that respond to neurotransmitters either by a direct action of G proteins on ion channels or by G-protein-enzyme activation that leads to formation of diffusible second messengers EPSP Excitatory postsynaptic potential; a depolarizing potential change IPSP Inhibitory postsynaptic potential; a hyperpolarizing potential change Synaptic mimicry Ability of an administered chemical to mimic the actions of the natural neurotransmitter: a criterion for identification of a putative neurotransmitter
Sites & Mechanisms of Drug Action
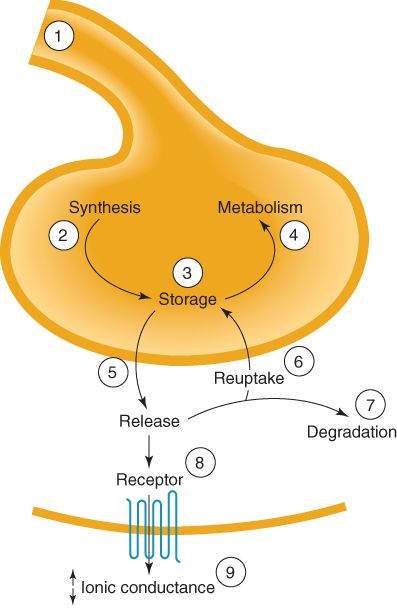
A small number of neuropharmacologic agents exert their effects through direct interactions with molecular components of ion channels on axons. Examples include certain anticonvulsants (eg, carbamazepine, phenytoin), local anesthetics, and some drugs used in general anesthesia. However, the effects of most therapeutically important CNS drugs are exerted mainly at synapses. Possible mechanisms are indicated in Figure 21-2. Thus, drugs may act presynaptically to alter the synthesis, storage, release, reuptake, or metabolism of transmitter chemicals. Other drugs can activate or block both pre- and postsynaptic receptors for specific transmitters or can interfere with the actions of second messengers. The selectivity of CNS drug action is largely based on the fact that different groups of neurons use different neurotransmitters and that they are segregated into networks that subserve different CNS functions.
FIGURE 21-2
Sites of CNS drug action. Drugs may alter (1) the action potential in the presynaptic fiber; (2) synthesis of transmitter; (3) storage ; (4) metabolism; (5) release; (6) reuptake; (7) degradation; (8) receptor for the transmitter; or (9) receptor-induced decrease or increase in ionic conduction.
(Reproduced, with permission, from Katzung BG, editor: Basic & Clinical Pharmacology, 10th ed. McGraw-Hill, 2007: Fig. 21-2.)
A few neurotoxic substances damage or kill nerve cells. For example, 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP) is cytotoxic to neurons of the nigrostriatal dopaminergic pathway.
Role of CNS Organization
The CNS contains 2 types of neuronal systems: hierarchical and diffuse.
Hierarchical Systems
These systems are delimited in their anatomic distribution and generally contain large myelinated, rapidly conducting fibers. Hierarchical systems control major sensory and motor functions. The major excitatory transmitters in these systems are aspartate and glutamate. These systems also include numerous small inhibitory interneurons, which use  -aminobutyric acid (GABA) or glycine as transmitters. Drugs that affect hierarchical systems often have profound effects on the overall excitability of the CNS.
-aminobutyric acid (GABA) or glycine as transmitters. Drugs that affect hierarchical systems often have profound effects on the overall excitability of the CNS.
Diffuse Systems
Diffuse or nonspecific systems are broadly distributed, with single cells frequently sending processes to many different areas. The axons are fine and branch repeatedly to form synapses with many cells. Axons commonly have periodic enlargements (varicosities) that contain transmitter vesicles. The transmitters in diffuse systems are often amines (norepinephrine, dopamine, serotonin) or peptides that commonly exert actions on metabotropic receptors. Drugs that affect these systems often have marked effects on such CNS functions as attention, appetite, and emotional states.
Transmitters at Central Synapses
Criteria for Transmitter Status
To be accepted as a neurotransmitter, a candidate chemical must (1) be present in higher concentration in the synaptic area than in other areas (ie, must be localized in appropriate areas), (2) be released by electrical or chemical stimulation via a calcium-dependent mechanism, and (3) produce the same sort of postsynaptic response that is seen with physiologic activation of the synapse (ie, must exhibit synaptic mimicry). Table 21-1 lists the most important chemicals currently accepted as neurotransmitters in the CNS.
TABLE 21-1 Neurotransmitter pharmacology in the CNS.
Transmitter Anatomical Distribution Receptor Subtypes Receptor Mechanisms Acetylcholine Cell bodies at all levels, short and long axons Muscarinic, M1; blocked by pirenzepine and atropine
Excitatory;  K+ conductance;
K+ conductance;  IP 3 and DAG
IP 3 and DAG
Muscarinic, M2; blocked by atropine
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree