and Miguel A. R. B. Castanho2
(1)
Instituto de Bioquímica Médica Leopoldo de Meis, Federal University of Rio de Janeiro, Rio de Janeiro, Rio de Janeiro, Brazil
(2)
Institute of Biochemistry and Institute of Molecular Medicine, School of Medicine, University of Lisbon, Lisbon, Portugal
Studying molecules is the key to understanding life. A commonly accepted definition of life, known as the NASA (North American Space Agency) hypothesis, states that “Life is a self-sustained chemical system capable of undergoing Darwinian evolution” (Fig. 1.1). The link between molecules and life may be hard to explain, but it is simple to illustrate.
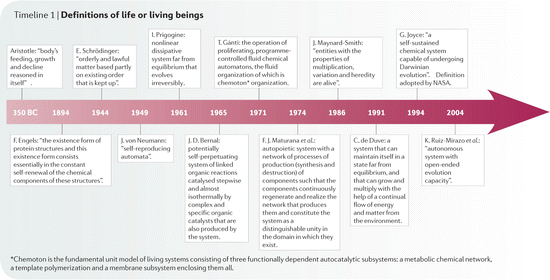

Fig. 1.1
Timeline for the definitions of life or living beings. Figure reproduced with permission from Moreva & López-Garcia, Nat. Rev. Microbiol. 7:306–311, 2009
In this introduction we have selected three examples that are sufficient to show that knowledge on molecules is essential to reason about life itself, health, and disease:
1.
Searching for the origin of life is a chemical “adventure” involving the molecules of primitive Earth and their reactivity.
2.
Viruses are amazing molecular machines, too simple to be considered living beings for most researchers, but with a tremendous ability to interfere with the course of life, sometimes tragically.
3.
The world of drug discovery and development consists of molecules being designed and synthesized and interacting with other molecules in silico, in vitro, and in vivo with the end goal of interfering with vital physiologic processes.
It is all about molecules. It is all about life.
1.1 Selected Illustrative Example #1: The Molecular Origin of Life
Nothing is better than trying to answer the question “what was the origin of life ?” to realize that molecules are the key to life. Since the pioneering work of Aleksandr Oparin, the origin and evolution of life are elucidated based on the chemistry of molecules containing carbon. By introducing this concept, Oparin truly revolutionized the way science interprets life. Nowadays, there are two main hypotheses to explain the evolution of the complexity of molecular organization into what one today calls cells, the so-called “replicator” and “metabolism ” hypotheses (Fig. 1.2). These hypotheses are based on two specific characteristics common to all living beings: Despite tremendous diversity among species, all life forms are organized in cells and all cells have a replicator polymer (DNA ) and a membrane with restricted permeability (a “membrane” having lipids in its composition). Therefore, it is not surprising that the prevailing hypotheses to explain the origin of life are indeed models that elaborate on the appearance of the replicator polymer and compartmentalization. The replicator polymer is essential to transmit the molecular information inherited from the previous generation, and a membrane forming a compartment that separates the ancestral cell from its environment is essential to ensure that the molecules in this space can react among each other in a controlled and self-regulated way (a “proto-metabolism”), with minimal impact of fluctuations in environmental conditions. These two aspects are consensual among researchers who study the origin of life, but the details and chronological order of events that resulted in cells as we know today is far from being established.
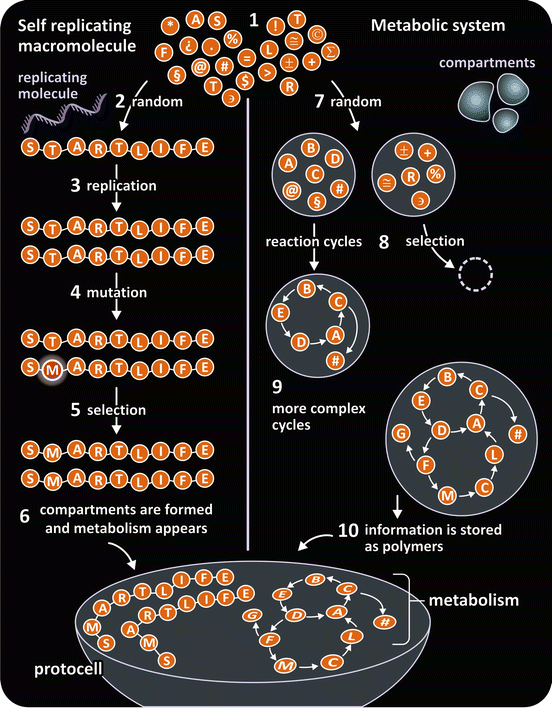

Fig. 1.2
Schematic representation of the replicator and metabolism hypotheses to describe the origin of life . Both models are molecular in nature and agree on the critical roles of a replicator molecule and compartmentalization but differ on the sequence of events. Figure reproduced with permission from Saphiro, Investigacion y Ciencia 371, 2007

1.1.1 The Replicator Hypothesis
According to the replicator hypothesis, life started with a molecule that was randomly formed but had the ability to replicate itself. It is an extremely unlikely event, hardly possible to occur twice in the universe, but one may work on the hypothesis that it has occurred. The obvious first “choice” for a replicator molecule is DNA , the ubiquitous replicator nowadays, but this leaves us in a paradox: proteins are needed to generate DNA and DNA is needed to generate proteins. What came first then? It may be that DNA had an ancestor with self-catalytic activity. RNA is eligible as such ancestor. RNA is not as chemically stable as DNA, so it is not so well suited to store information for long periods of time, but it can still constitute genetic material (many viruses, such as HIV or dengue virus, have RNA genomes). Concomitantly, RNA conformation dynamics enables catalytic activity, a perfect combination for the original replicator. The introduction of mutations and other errors in replication, in addition to other mechanisms, led to evolution and selection. How this process was coupled to the appearance of a metabolism is hard to conceive, but confinement of replicators into separated environments may have favored some chemical reactions that evolved in their restrained space to cause metabolism (Fig. 1.2).
1.1.2 The Metabolism Hypothesis
An alternative model skips the Achilles heel of the replicator hypothesis. Here, the origin of life in not dependent on a starting event that is nearly impossible to succeed. The key process was the confinement of small molecules that reacted among them. In some cases, organized ensembles of molecules may have formed stable reaction cycles that became increasingly complex. The result was the creation of metabolism and complex polymer molecules, including replicators (Fig. 1.2). Naturally, the boundaries of the confined environment where these reactions took place had to allow for selective permeation of matter. Permeation allowed growth and replication.
Nowadays, virtually all cell membranes are formed non-exclusively but mostly by lipids . Modern lipids are synthetic products of metabolism . So what could have been the predecessors of lipid membranes in the confinement of the first “proto-metabolic” reactions? Orevices in the outer layers of rocks are a possibility. Phospholipids or other surfactant molecules may have started as coatings that, due to their intrinsic dynamics and capability to expand into a film and seal, may have evolved into membranes. Lipids and other surfactants have the ability to form three-dimensional structures other than lamellae that may have contributed to confine chemical systems (Fig. 1.3).
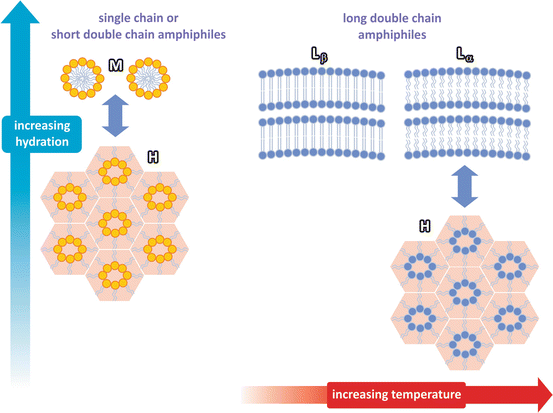

Fig. 1.3
The structure of lipid assemblies depends mainly on the degree of hydration and the molecular structure of lipids . Lipids may organize in different ways: rigid bilayers (Lβ), fluid bilayers (Lα), micelles (M), or hexagonal (H) phases
Metabolism evolved towards self-regulation creating homeostasis, a situation whereby a balance exists. Small to moderate perturbations of such balance trigger responses that tend to reestablish the original, equilibrated balance. The ability of certain metabolites (intermediate molecules in a complex sequence of reactions in a living system) to activate or inhibit specific reactions in the metabolism was a major contribution to homeostasis (Fig. 1.4).
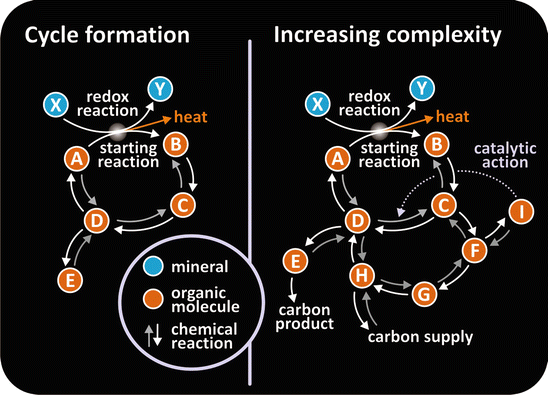

Fig. 1.4
The evolution of networks of chemical reactions. Simple cycles of reactions (left) may have evolved in complexity (right). The interference of certain metabolites on the course of reactions possibly resulted in self-regulated metabolisms . Figure reproduced with permission from Saphiro, Investigacion y Ciencia 371, 2007
Nowadays, even the simplest cells, mycoplasma bacteria, for example, are extremely complex systems from the chemical/molecular point of view. Considering natural evolution, all metabolisms in all living cells are related by historical bonds and “metabolic maps” showing the main metabolic sequences in living beings can be drawn (Fig. 1.5). It is amazing that these complex series of reactions operate and do not conflict with each other. In reality, not all reactions depicted in (Fig. 1.5) occur in the same species and the ones that do occur in the same species may not be present in all cells. In case they co-exist in the same cell, they may not occur in the same cell compartment and in case they do, they may not be working at the same time. “Complex” does not mean “confusing.”
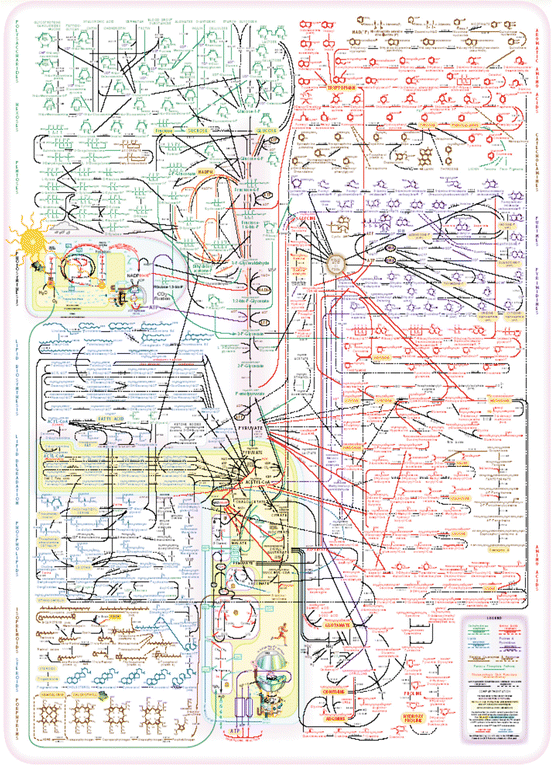

Fig. 1.5
A metabolic map showing a hypothetical cell, where the whole metabolism would gather many different sectorial metabolisms : amino acid s , phospholipids , steroids, lipids , saccharides , etc. In reality, not all cells perform all sectorial metabolisms; those that occur in a certain cell may not occur in the same organelle and those that occur in the same organelle may not occur at the same time. The metabolism as a whole is usually so complex that in practice one tends to refer to “metabolisms” referring to the sectorial metabolisms in short. The word may be misleading because it may leave the impression that there are several independent metabolisms. Metabolisms are not independent of each other and they are highly correlated, even those occurring in different organs. The need for metabolic regulation extends to the whole human body. Figure reproduced with permission of IUBMB, International Union of Biochemistry and Molecular Biology
Because metabolic pathway s (sets of metabolic reactions) have evolved from the same historical background, all molecules in all living cells are also related by historical links. Their common roots determined that, despite all the apparent molecular diversity, nearly all molecules in all cells can be grouped in few families. It is also intriguing at first glance that with so many chemical elements known to man (Fig. 1.6), cells rely heavily on very few of them: hydrogen, oxygen , carbon, and nitrogen are 99 % of the atoms that make a cell. How can this apparent puzzle be explained? Essentially, it all resorts to the common ancestor of all living cells in all living world: these were the most abundant elements in solution in the primitive ocean. These were the founding resources and life evolved from them.
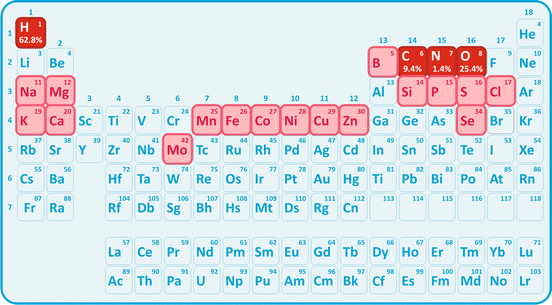

Fig. 1.6
Periodic chart of the elements, stressing the abundance of some in living beings (highlighted red). It should be noticed that very few elements are needed to “build” almost the totality of cells, and some elements are only present in trace amounts (highlighted pink). Yet, the elements that are rare may be absolute essential to life. Cobalt, Co, for instance, is part of vitamin B12 (see Box 3.8)
We shall revisit in a more detailed manner the chemical nature of cells in Chap. 3.
1.2 Selected Illustrative Example #2: Viruses, Molecular Machines Interfering with Life
Viruses are not considered by most researchers as living beings. They are on the boundary between living and non-living, able to interfere with homeostasis. They have similar molecular constituents compared to cells (proteins , lipids , nucleic acids, etc.), but there are important differences. Above all, viruses lack a metabolism of their own. Their simplicity is not a consequence of ancestry nor does it relate to any surviving form of primitive life. Instead, it is a consequence of parasitism and regressive evolution. Alternatively, viruses may have been part of the cells. Minimal genome sizes imply faster reproduction rates for viruses and are therefore an evolutionary advantage. One may argue that viruses lack a metabolism of their own but are physical entities that are able to self-replicate and evolve, thus living beings. Even so, it is questionable whether they may be considered as living because they do not replicate or evolve independently of cells. Virtually all parasites need a host to survive and multiply, but viruses are also not able to evolve independently: they are dependent on cells to evolve because they do not have their own machinery of molecular synthesis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


