2. The context-sensitive half-time of propofol is only minimally influenced by the duration of the infusion (when used as a sedative for prolonged intensive care unit [ICU] care, the context sensitive half-time is highly relevant).
3. Despite the rapid clearance of propofol by metabolism, there is no evidence of impaired elimination in patients with cirrhosis of the liver.
D. Clinical Uses
1. Induction of Anesthesia. The induction dose of propofol in healthy adults is 1.5 to 2.5 mg/kg IV (elderly patients require a 25% to 50% lower induction dose). Complete awakening without residual CNS effects is characteristic of propofol.
2. Intravenous Sedation
a. The short context-sensitive half-time of propofol, combined with the short effect-site equilibration time, make this a readily titratable drug for production of IV sedation. Prompt recovery without residual sedation and low incidence of nausea and vomiting make propofol particularly well suited to ambulatory conscious sedation techniques. The typical conscious sedation dose of 25 to 100 μg/kg/minute IV produces minimal analgesic and amnestic effects. In selected patients, midazolam or an opioid may be added to propofol for continuous IV sedation.
b. Propofol has emerged as the agent of choice for sedation for brief gastrointestinal endoscopy procedures. SEDASYS is a computer-assisted delivery system to provide sedation for upper endoscopy and colonoscopy.
c. Increasing metabolic acidosis, lipemic plasma, bradycardia, and progressive myocardial failure has been described.
3. Maintenance of Anesthesia. The typical dose of propofol for maintenance of anesthesia is 100 to 300 μg/kg/minute IV, often in combination with a short-acting opioid. General anesthesia that includes propofol is typically associated with minimal postoperative nausea and vomiting, and awakening is prompt, with minimal residual sedative effects.
E. Nonhypnotic Therapeutic Applications
1. Antiemetic Effects. The incidence of postoperative nausea and vomiting is decreased when propofol is administered, regardless of the anesthetic technique. When administered to induce and maintain anesthesia, it is more effective than ondansetron in preventing postoperative nausea and vomiting. Subhypnotic doses of propofol that are effective as an antiemetic do not inhibit gastric emptying and propofol is not considered a prokinetic drug.
2. Antipruritic Effects. Propofol, 10 mg IV, is effective in the treatment of pruritus associated with neuraxial opioids or cholestasis.
3. Anticonvulsant Activity. Propofol possesses antiepileptic properties, presumably reflecting GABA-mediated presynaptic and postsynaptic inhibition of chloride ion channels (doses >1 mg/kg IV decreases seizure duration 35% to 45% in patients undergoing electroconvulsive therapy).
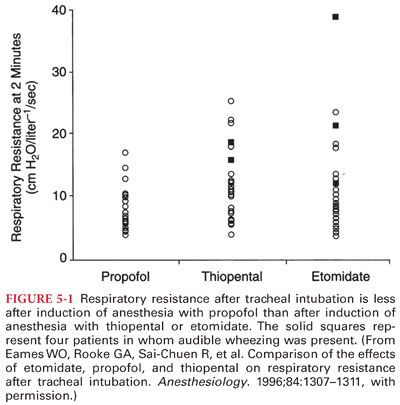
4. Attenuation of Bronchoconstriction. Propofol decreases the prevalence of wheezing after induction of anesthesia and tracheal intubation in healthy and asthmatic patients (Fig. 5-1). However, a formulation of propofol that uses metabisulfite as a preservative may cause bronchoconstriction in asthmatic patients.
F. Effects on Organ Systems
1. Central Nervous System
a. Propofol decreases cerebral metabolic rate for oxygen (CMRO2), cerebral blood flow, and intracranial pressure (ICP).
b. Cortical somatosensory evoked potentials as used for monitoring spinal cord function are not significantly modified in the presence of propofol alone but the addition of nitrous oxide or a volatile anesthetic results in decreased amplitude.
c. Development of tolerance to drugs that depress the CNS is a common finding, occurring with repeated exposure to opioids, sedative-hypnotic drugs, ketamine, and nitrous oxide (tolerance to propofol does not develop in children undergoing repeated exposure to the drug during radiation therapy).
2. Cardiovascular System
a. Propofol produces decreases in systemic blood pressure (Fig. 5-2). The relaxation of vascular smooth muscle produced by propofol is primarily due to inhibition of sympathetic vasoconstrictor nerve activity.
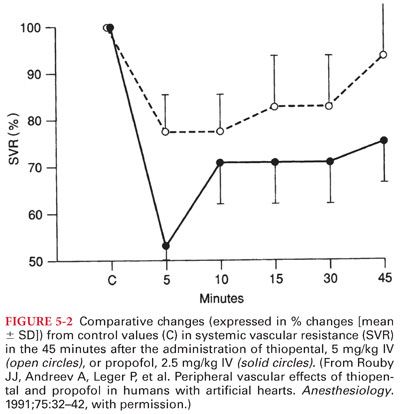
b. The blood pressure effects of propofol may be exaggerated in hypovolemic patients, elderly patients, and patients with compromised left ventricular function. Adequate hydration before rapid IV administration of propofol is recommended to minimize the blood pressure reduction.
c. Bradycardia-Related Death. Profound bradycardia and asystole after administration of propofol have been described in healthy adult patients, despite prophylactic anticholinergics (risk of bradycardia-related death during propofol anesthesia has been estimated to be 1.4 in 100,000). Treatment of propofol-induced bradycardia may require treatment with a direct β-agonist such as isoproterenol.
3. Lungs. Propofol produces dose-dependent depression of ventilation, with apnea occurring in 25% to 35% of patients after induction of anesthesia with propofol. Opioids administered with the preoperative medication enhance ventilatory depressant.
4. Hepatic and Renal Function. Propofol does not normally affect hepatic or renal function as reflected by measurements of liver transaminase enzymes or creatinine concentrations.
a. Prolonged infusions of propofol may also result in excretion of green urine, reflecting the presence of phenols in the urine (does not alter renal function).
b. Urinary uric acid excretion is increased after administration of propofol and may manifest as cloudy urine when the uric acid crystallizes in the urine under conditions of low pH and temperature (not considered to be detrimental).
5. Intraocular Pressure. Propofol is associated with significant decreases in intraocular pressure that occur immediately after induction of anesthesia and are sustained during tracheal intubation.
6. Coagulation. Propofol inhibits platelet aggregation that is induced by proinflammatory lipid mediators including thromboxane A2 and platelet-activating factor.
7. Allergic Reactions. Patients who develop evidence of anaphylaxis on first exposure to propofol may have been previously sensitized to the diisopropyl radical, which is present in many dermatologic preparations. Anaphylaxis to propofol during the first exposure to this drug has been observed, especially in patients with a history of other drug allergies, often to neuromuscular blocking drugs.
8. Lactic acidosis (“propofol infusion syndrome”) has been described in pediatric and adult patients receiving prolonged high-dose infusions of propofol (>75 μg/kg/minute) for longer than 24 hours.
a. The mechanism for sporadic propofol-induced metabolic acidosis is unclear but may reflect poisoning (cytopathic hypoxia) of the electron transport chain and impaired oxidation of long chain fatty acids by propofol or a propofol metabolite in uniquely susceptible patients (mimics the mitochondrial myopathies).
b. The differential diagnosis when propofol-induced lactic acidosis is suspected includes hyperchloremic metabolic acidosis associated with large volume infusions of 0.9% saline and metabolic acidosis associated with excessive generation of organic acids, such as lactate and ketones (diabetic acidosis, release of a tourniquet). Measurement of the anion gap and individual measurements of anions and organic acids will differentiate hyperchloremic metabolic acidosis from lactic acidosis.
9. Proconvulsant Activity. The majority of reported propofol-induced “seizures” during induction of anesthesia or emergence from anesthesia reflect spontaneous excitatory movements of subcortical origin (not thought to be due to cortical epileptic activity). There appears to be no reason to avoid propofol for sedation, induction, and maintenance of anesthesia in patients with known seizures.
10. Abuse Potential. Intense dreaming activity, amorous behavior, and hallucinations have been reported during recovery from and low-dose infusions of propofol. Addiction has been described.
11. Bacterial Growth
a. Propofol strongly supports the growth of Escherichia coli and Pseudomonas aeruginosa. Postoperative surgical infections manifesting as temperature elevations have been attributed to extrinsic contamination of propofol. For this reason, it is recommended that (a) an aseptic technique be used in handling propofol as reflected by disinfecting the ampule neck surface or vial rubber stopper with 70% isopropyl alcohol; (b) the contents of the ampule containing propofol should be withdrawn into a sterile syringe immediately after opening and administered promptly; and (c) the contents of an opened ampule must be discarded if they are not used within 6 hours.
b. Despite these concerns, there is evidence that when propofol is aseptically drawn into an uncapped syringe, it will remain sterile at room temperature for several days.
12. Antioxidant Properties. Propofol has potent antioxidant properties that resemble those of the endogenous antioxidant vitamin E (neuroprotective effect of propofol).
13. Pain on injection is the most commonly reported adverse event associated with propofol administration to awake patients. Preceding the propofol with 1% lidocaine or prior administration of a potent short-acting opioid decreases the incidence of discomfort experienced by the patient.
14. Miscellaneous Effects
a. Propofol does not trigger malignant hyperthermia.
b. Secretion of cortisol is not influenced by propofol.
III. Etomidate is a carboxylated imidazole–containing compound that is chemically unrelated to any other drug used for the IV induction of anesthesia.
A. Commercial Preparation
1. The original formulation of etomidate (high incidence of pain during IV injection) has been changed to a fat emulsion which has virtually abolished pain on injection and venous irritation, whereas the incidence of myoclonus remains unchanged.
2. An oral formulation of etomidate for transmucosal delivery has been shown to produce dose-dependent sedation. Administration through the oral mucosa results in direct systemic absorption while bypassing hepatic metabolism (higher blood concentrations are achieved more rapidly compared with drug that is administered by mouth).
B. Mechanism of Action. The anesthetic effect of etomidate resides predominantly in the R(+) isomer, which is approximately five times as potent as the S(–) isomer. Stereoselectivity of etomidate supports the concept that GABAA receptors are the site of action of etomidate.
C. Pharmacokinetics Uptake (see Table 5-1). Prompt awakening after a single dose of etomidate principally reflects the redistribution of the drug from brain to inactive tissue sites. Rapid metabolism is also likely to contribute to prompt recovery.
D. Metabolism. Etomidate is rapidly metabolized by hydrolysis of the ethyl ester side chain to its carboxylic acid ester, resulting in a water-soluble, pharmacologically inactive compound.
E. Clinical Uses
1. Etomidate may be viewed as an alternative to propofol or barbiturates for the IV induction of anesthesia, especially in the presence of an unstable cardiovascular system. After a standard induction dose of 0.2 to 0.4 mg/kg IV, the onset of unconsciousness occurs within one arm-to-brain circulation time.
2. Involuntary myoclonic movements are common during the induction period as a result of alteration in the balance of inhibitory and excitatory influences on the thalamocortical tract (frequency of this myoclonic-like activity can be attenuated by prior administration of an opioid).
3. The principal limiting factor in the clinical use of etomidate for induction of anesthesia is the ability of this drug to transiently depress adrenocortical function.
F. Side Effects
1. Central Nervous System
a. Etomidate is a potent direct cerebral vasoconstrictor that decreases cerebral blood flow and CMRO2 35% to 45% (previously increased ICP is lowered by etomidate). Suppression of adrenocortical function limits the clinical usefulness for long-term treatment of intracranial hypertension.
b. Etomidate may activate seizure foci, manifesting as fast activity on the electroencephalogram (EEG) (use with caution in patients with focal epilepsy). This characteristic may be used to facilitate localization of seizure foci in patients undergoing cortical resection of epileptogenic tissue. Etomidate also possesses anticonvulsant properties and has been used to terminate status epilepticus.
c. Etomidate has been observed to augment the amplitude of somatosensory evoked potentials, making monitoring of these responses more reliable.
2. Cardiovascular System
a. Cardiovascular stability is characteristic of induction of anesthesia with 0.3 mg/kg IV of etomidate (minimal changes in heart rate, stroke volume, or cardiac output, whereas mean arterial blood pressure may decrease up to 15% because of decreases in systemic vascular resistance).
b. Etomidate has been proposed for induction of anesthesia in patients with little or no cardiac reserve. Etomidate may differ from most other IV anesthetics in that depressive effects on myocardial contractility are minimal at concentrations needed for the production of anesthesia.
3. Ventilation. The depressant effects of etomidate on ventilation seem to be less than those of barbiturates, although apnea may occasionally accompany a rapid IV injection of the drug. In the majority of patients, etomidate-induced decreases in tidal volume are offset by compensatory increases in the frequency of breathing.
4. Pain on injection and venous irritation has been virtually eliminated with use of etomidate in a lipid emulsion vehicle rather than propylene glycol.
5. Myoclonus
a. Commonly administered IV anesthetics can cause excitatory effects that may manifest as spontaneous movements, such as myoclonus, dystonia, and tremor. These spontaneous movements, particularly myoclonus, occur in 50% to 80% of patients receiving etomidate in the absence of premedication. Prior administration of an opioid (fentanyl, 1 to 2 μg/kg IV) or a benzodiazepine may decrease the incidence of myoclonus associated with administration of etomidate.
b. The mechanism of etomidate-induced myoclonus appears to be disinhibition of subcortical structures that normally suppress extrapyramidal motor activity.
6. Adrenocortical Suppression
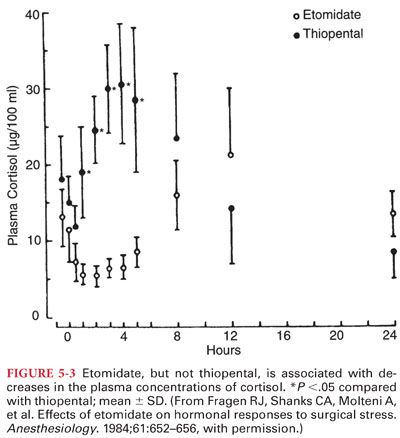
a. Etomidate causes adrenocortical suppression by producing a dose-dependent inhibition of the conversion of cholesterol to cortisol (Fig. 5-3). The specific enzyme inhibited by etomidate appears to be 11-β-hydroxylase (enzyme inhibition lasts 4 to 8 hours after an induction dose of etomidate).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


