2.1 Introduction
Pharmacologically active substances have been administered to humans for thousands of years, but delivery via the lung is a relatively recent development. Commercialization of inhaled respiratory medicines was not achieved until 1948, when Abbot Laboratories developed the Aerohaler for inhaled penicillin G powder; they then revolutionized the field in 1955 with the advent of the pressurized metered-dose inhaler (pMDI) [1]. Since this inception, the range of inhaler products and medicaments has grown to encompass alternative drug delivery systems, namely those based on the dry powder inhaler (DPI), nebulizers, and nasal products. This chapter will review the three main inhalation delivery platforms (DPIs, pMDIs, and nebulizers), as well as nasal drug-delivery methods and formulations.
2.2 Dry Powder Inhalers (DPIs)
DPIs, as their name implies, contain and deliver the active medicament as a dry powder of suitable aerodynamic size for respiratory therapy. Dry powder particles of a suitable size, generally less than 6 microns [2], can be readily produced by micronization or by other size-reduction methods. However, such particles have high surface area to mass ratios, making them highly cohesive/adhesive. Consequently, the drug must be formulated in such a way that the energy input during inhalation is sufficient to overcome the contiguous adhesive and cohesive particle forces, aerosolizing the powder for respiratory deposition. Although in principle this approach may appear straightforward, the physicochemical nature and interactive mechanisms of the components in a DPI system are still relatively poorly understood. Many commercial products have, by pharmaceutical standards, relatively poor efficiencies, with often less than 20% drug being delivered to the lung [3]. This has generated a significant amount of research activity in the fields of pharmaceutics, powder technology, and surface, aerosol, and colloid science, which has focused on understanding the interactions in DPI systems.
The specific technologies underpinning DPI formulation are discussed in Chapter 3, but basically the efficacy of a DPI formulation is dependent on both the formulation of the powder and the design of the device. There are two main approaches to formulating the drug powder:
Examples of carrier-based systems include GlaxoSmithKline’s (GSK’s) Ventolin Diskus and Novartis’s Foradil Aeroliser products. Examples of agglomerated drug-only formulations include Astra Zeneca’s (AZ’s) Pulmicort and Bricanil Turbohalers. A company product will come with a formulation (drug name) and a device name. Some formulations are reformulated in multiple devices (for example, GSK’s Ventolin has been available in both the Diskhaler and Diskus devices).
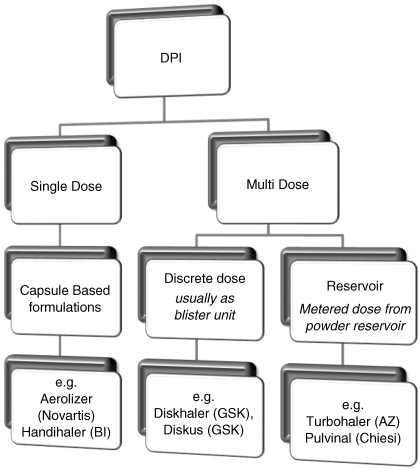
In terms of design, DPI devices are either single-dose systems (which require refilling by the patient) or multi-dose systems (where the patient activates the dose pre-inhalation). Single-dose systems usually utilize capsules to store the formulation, which are inserted into the device, and pierced by the patient, prior to inhalation. Multi-dose systems store the powder either in individual blisters (which are fired sequentially) or in a powder reservoir (which is metered by the device). A chart outlining the different forms of device, along with examples, is given in Figure 2.1.
An important thing to note with commercially available DPIs is that they are all passive devices; passive devices rely on the patients inspiratory flow rate to generate the energy for powder dispersion. It is therefore important for inhaler developers to design devices that (1) allow powder dispersion upon inhalation at reasonable flow rates and (2) have flow-rate independence. It is important to note that different patients will have different lung capacities and maximum ventilatory flows (according to their demographic and disease severity), and thus variation in device performance and resistance with respect to flow is critical to consistent efficacy [4].
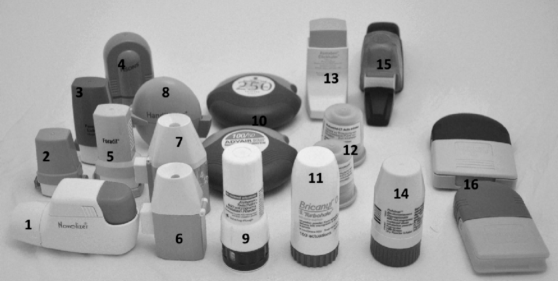
DPI devices have been around for more than 20 years and thus a wide range of devices have been or are currently available on the market. A collection of these devices is shown in Figure 2.2.
Figure 2.2 Examples of past and present DPI devices. (1) Novolizer by Almirall S. A., (2) Aerolizer. The AEROLIZER® photo image is reproduced with permission of Merck Sharp & Dohme Corp., subsidiary of Merck & Co., Inc. All rights reserved. The trademark AEROLIZER® is a registered trademark of Novartis AG., (3) Cyclohaler Courtesy of Teva UK Ltd., (4) Spinhaler by Rhodia (Fisons), (5) Aerolizer. The AEROLIZER® photo image is reproduced with permission of Merck Sharp & Dohme Corp., subsidiary of Merck & Co., Inc. All rights reserved. The trademark AEROLIZER® is a registered trademark of Novartis AG., (6) Inhalateur Ingelheim. Courtesy of Boehringer Ingelheim Ltd., (7) Aerohaler/Inhalator. Courtesy of Boehringer Ingelheim Ltd., (8) Handihaler. Courtesy of Boehringer Ingelheim Ltd., (9) Twisthaler. The TWISTHALER® photo image is reproduced with permission of Merck Sharp & Dohme Corp, subsidiary of Merck & Co., Inc. All rights reserved. The trademark TWISTHALER® is a registered trademark of Merck Sharp & Dohme Corp., (10) Accuhaler/Diskus by GlaxoSmithKline Respiratory, (11) Turbohaler. Courtesy of AstraZeneca UK Ltd., (12) Auto-Jethaler/Auto-haler by RatioPharm (PulmoTec), (13) Clickhaler. Courtesy of Innovata Biomed Ltd., (14) Pulvinal. Courtesy of Chiesi Farmaceutici, Parma, Italy, (15) Easyhaler by Orion Pharma, (16) Diskhaler by GlaxoSmithKline Respiratory (Allen & Hanburys)
Another important issue to consider regarding DPI devices is their ease of use. The evolution of DPI devices has come about, in part, due to coordination issues associated with their pMDI counterparts. However, while DPI devices are relatively free from coordination issues, device complexity must be considered when prescribing medicines. Many of the first-generation and some of the second-generation DPI devices require multiple steps to ensure a dose is loaded and/or may require specific cleaning routines after use (Table 2.1 shows the Relenza operating instructions, for example). The former point must be considered when selecting a device for patients who have potential dexterity issues. In addition, when introducing new or alternative devices to a patient, which have open reservoirs or are moisture-sensitive, storage (in a bathroom cabinet, for example) must be discussed in order to avoid degradation of the formulation. It is important to ensure that the prescription of a new device and formulation is accompanied by adequate education from the general practitioner, consultant, and pharmacist.
Table 2.1 Example of a patient instruction leaflet for the RS01 DPI inhaler (Plastiape, IT) .
| Correct Use of RS01 DPI Inhaler (Plastiape, IT) | |
1. Remove the cap. 2. Hold the inhaler at base and turn the mouthpiece in anticlockwise direction. 3. Place a capsule in the compartment in base of the inhaler. 4. Twist the mouthpiece to closed position. 5. Hold the inhaler upright, squeezing the two blue buttons inwards on the base of inhaler to pierce the capsule, then release. 6. Breathe out fully. 7. Insert the mouthpiece into your mouth, sealing lips and teeth around mouthpiece. 8. Inhale quickly and deeply. 9. Hold your breath for count of ten, or as long as is comfortable. 10. Breathe out gently. 11. Discard capsule from the compartment in the base of the inhaler. 12. Replace the cap. |  |
2.3 Liquid and Propellant-Based Inhalers
Liquid and propellant aerosol systems can be generally subclassified into nebulizer and pMDI technologies. The main difference between the two is that nebulizers utilize an external energy source to produce aerosolized fine particulate droplets of the formulation while pMDI systems incorporate a propellant into the formulation, which provides the energy for aerosolization upon actuation. Both of these approaches have advantages and disadvantages, both can contain either solution- or suspension-based formulation strategies, and both have undergone technological advances in recent years.
2.3.1 Pressurized Metered-Dose Inhalers (pMDIs)
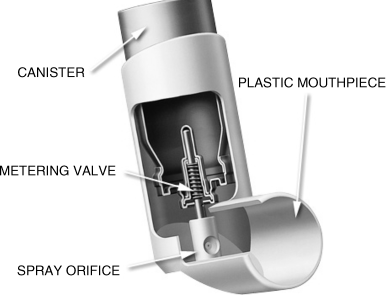
pMDIs (or MDIs in the USA) were introduced in the 1950s by Riker Laboratories [5]. Initially the formulation was contained in a chlorofluorocarbon (CFC) propellant, but this was superseded by hydrofluoroalkane (HFA) propellants (between the 1990s and early 2000s) due to the Montreal Protocol ban on the former, ozone-damaging molecules [6]. The canister is typically a formed single aluminum component with a metering valve, crimped to the canister lid. The metering valve isolates the pressurized formulation’s local environment (typically 4–5 atmospheres of pressure) from the surrounding environment (1 atmosphere), and upon actuation releases a known quantity of propellant formulation (typically 25–100 μL) to the surrounding environment. The formulation is either solubilized or suspended in the propellant, and the local concentration of drug in the canister determines the therapeutic dose. Expansion of the propellant as it exits the spray orifice results in a fine mist of HFA, which evaporates off to leave a micron-sized drug system suitable for inhalation. A schematic of the main components of a pMDI is shown in Figure 2.3.
The advantages of pMDI-based inhalation systems are their portable nature, ease of operation, and familiarity (although this may not necessarily be an advantage, since familiarity does not necessarily go hand-in-hand with correct use). In terms of disadvantages, pMDI devices have a smaller delivery volume than their DPI and nebulizer counterparts (limiting the range of therapeutic doses). Furthermore, some patients complain of a cooling “Freon” effect, due to the rapid evaporation of the propellant [7]. However, the most important patient-related issue with respect to these devices is the requirement for a coordinated inhalation and actuation maneuver. The pMDI is an active device, delivering a single dose over a period of microseconds. In comparison to nebulizers (which patients inhale over minutes of tidal breathing) or DPIs (which rely on the patient’s inspiratory flow to aerosolize the powder), the inhalation of pMDIs requires a coordinated effort during actuation. If the patient inhales before or after actuation, the majority of the medicament is likely to impact in the throat and be swallowed.
To overcome such issues, two approaches have been taken: spacers and breath-actuated pMDIs. Both attempt to overcome issues with poor patient coordination and have advantages and disadvantages.
Spacers are add-ons which attach to the mouthpiece of the pMDI. The patient or care worker actuates the device, delivering the aerosol into the spacer, so that the patient can inhale via tidal breathing (Figure 2.4a).
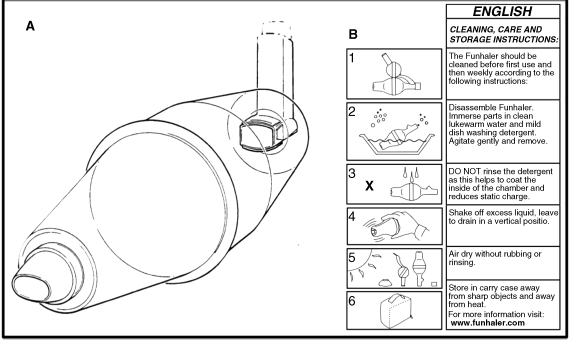
Figure 2.4 (a) Schematic of the GSK Volumatic Spacer device by GlaxoSmithKline. (b) Instruction leaflet for the Avita Funhaler. Image modified from Rotherham General Hospitals NHS Trust. Courtesy of Avita Medical Ltd
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree