Inflammatory and Reactive Tumors
FAT NECROSIS
Fat necrosis may result from incidental trauma, but, presently, the most frequent causes are prior surgery and radiation therapy. Patients typically present with a painless mass located superficially in the breast, accompanied by retraction or dimpling of the overlying skin. Any part of the breast may be affected. The tumors average 2 cm. The clinical problem of distinguishing between fat necrosis and recurrent carcinoma is especially difficult in patients who have undergone breast-conserving surgery and radiation therapy (1). Fat necrosis has been reported after external beam therapy and at the site of iridium implantation (2,3). Hemorrhagic necrosis of the skin, subcutaneous tissue, and breast parenchyma associated with Coumadin anticoagulant treatment is an uncommon form of fat necrosis (4). Pain and breast swelling appear within a week of the start of therapy, usually progressing to gangrene of most or all of the breast (5).
Mammography of fat necrosis usually reveals a spiculated mass that may contain punctate or large irregular calcifications (6). Less frequently, the lesion consists of a circumscribed, oil-filled, partly calcified cyst (7). Both patterns may coexist in a single lesion.
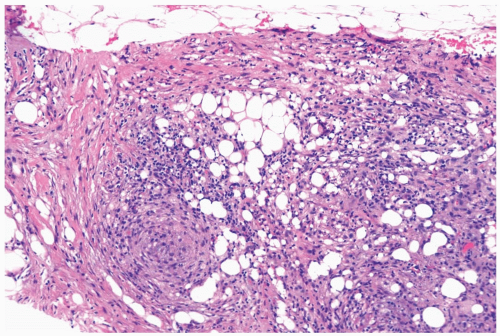
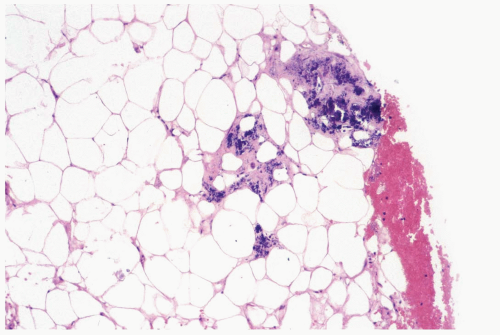
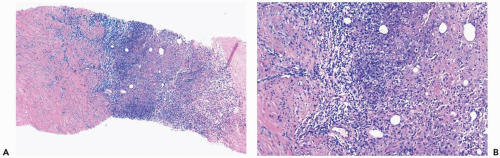
The initial histologic change in fat necrosis is disruption of fat cells and hemorrhage (Fig. 2.1). Evolution of the lesion is marked by the appearance of histiocytes, hemosiderin deposition, and a variable infiltrate of lymphocytes, plasma cells, and sometimes eosinophils (Fig. 2.2). A foreign body, giant cell reaction may be elicited (Fig. 2.3). Fibrosis develops peripherally, demarcating the area of necrotic fat, cellular debris, and calcifications (Fig. 2.4). In late lesions, the reactive inflammatory components are replaced by fibrosis and contract into a scar. Loculated, necrotic fat with calcification may persist for months or years within such a scar (Fig. 2.5). Squamous metaplasia may develop in the epithelium of ducts and lobules in the vicinity of fat necrosis.
A biopsy is required in most instances because the clinical and radiologic features resemble those of carcinoma. When there is a history of trauma or prior surgery and characteristic radiologic findings of a demarcated lesion with typical calcifications, excision may not be performed after the diagnosis has been established by needle core biopsy.
BREAST INFARCT
The most frequent form of breast infarct occurs during pregnancy or postpartum. The lesion presents as a discrete mass that can clinically suggest carcinoma. Pain and tenderness are sometimes reported. A firm mass is produced by infarcted mammary parenchyma. Hemorrhage and ischemic degeneration, with little or no inflammation, characterizes the histologic appearance of early lesions. Later stages feature fully developed coagulative necrosis with pallor, loss of nuclear detail, and loss of architectural integrity.
Infarction also occurs spontaneously in fibroadenomas and benign proliferative lesions. Foci of necrosis may be found in florid sclerosing adenosis, usually during pregnancy, when the epithelium in sclerosing adenosis may also exhibit pronounced hyperplasia, cytologic atypia, and mitotic activity. Papillomas are susceptible to partial or complete infarction, especially in major lactiferous ducts. Infarction can occur in papillomas at any age, but it tends to be more frequent in postmenopausal women, and there is no association with pregnancy (8). Bloody nipple discharge is the most frequent sign of an infarcted or degnerated papilloma.
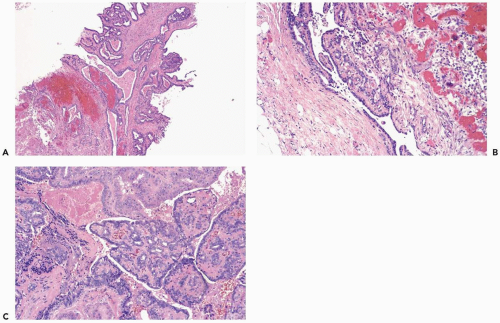
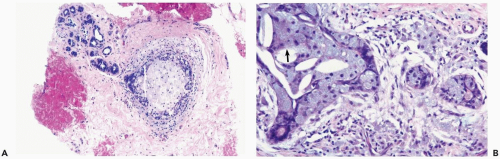
Acutely infarcted regions in a papilloma exhibit ischemic coagulative necrosis microscopically. Structural integrity is usually maintained in such foci, despite progressive loss of cytologic detail (Fig. 2.6). At a late stage, fragmentation of superficial portions of infarcted regions occurs. Mitotic activity may be present in intact epithelium
around the infarcted region in a papilloma. Occasionally, an infarcted papilloma is reduced to an inflammatory, intraductal polyp consisting of granulation tissue, with little or no epithelium. Chronic ischemia and healing of infarcts are marked by fibrosis, which may cause considerable distortion of residual entrapped epithelium, producing a pattern that could be mistaken for carcinoma (8). Squamous metaplasia sometimes develops in the reparative epithelium that proliferates after infarction, and calcification may form in the infarcted tissue (8,9). Sometimes infarcted carcinoma can be distinguished from infarction of a benign lesion, if there is residual intact in situ or invasive carcinoma (10).
around the infarcted region in a papilloma. Occasionally, an infarcted papilloma is reduced to an inflammatory, intraductal polyp consisting of granulation tissue, with little or no epithelium. Chronic ischemia and healing of infarcts are marked by fibrosis, which may cause considerable distortion of residual entrapped epithelium, producing a pattern that could be mistaken for carcinoma (8). Squamous metaplasia sometimes develops in the reparative epithelium that proliferates after infarction, and calcification may form in the infarcted tissue (8,9). Sometimes infarcted carcinoma can be distinguished from infarction of a benign lesion, if there is residual intact in situ or invasive carcinoma (10).
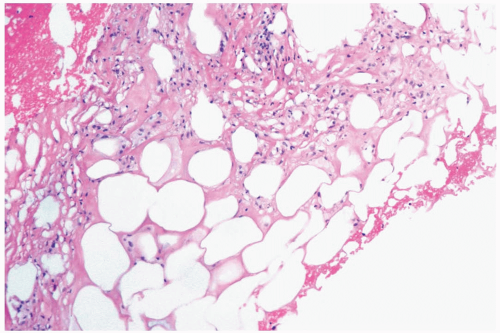 Figure 2.1 Fat necrosis. The needle core biopsy specimen obtained from a 1-cm stellate lesion consists of infarcted fat cells, hemorrhage, and histiocytic reaction. |
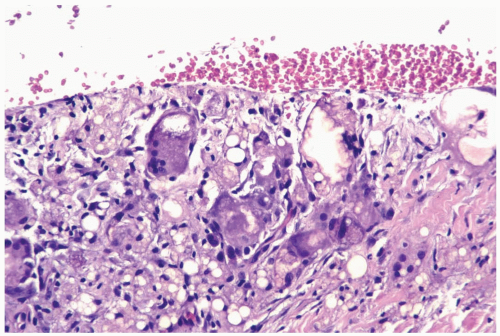 Figure 2.3 Fat necrosis. Multinucleated histiocytes with foreign body material are shown in a needle core biopsy specimen from fat necrosis at a prior surgical site. |
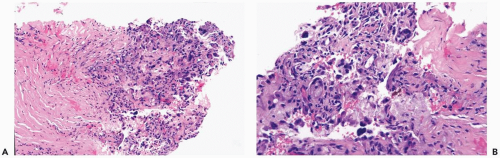 Figure 2.4 Fat necrosis. A, B. Fibrosis is evident around this focus of long-standing fat necrosis in a needle core biopsy specimen. |
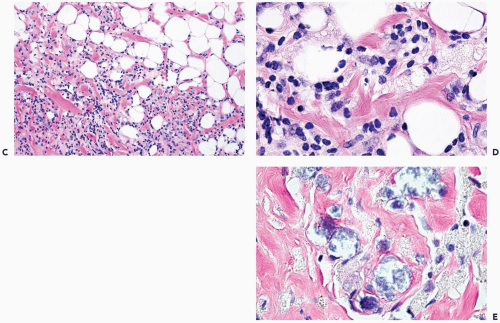 Figure 2.4 (continued) C, D. Lymphocytes and histiocytes in fat necrosis from a 52-year-old woman. E. Calcifications in the lesion shown in (C) and (D). |
The reliability of immunostains for evaluating infarcted papillary lesions is unpredictable and probably depends on the extent of decomposition. In some instances, immunoreactivity for cytokeratin and myoepithelial markers, especially p63, is surprisingly well preserved. When this occurs it may be possible to “resurrect” the structure of the lesion to a considerable degree.
Excisional biopsy is usually necessary for the diagnosis of a mammary infarct, although the findings in a needle core biopsy specimen may be suggestive. In most cases, recognition of the underlying condition hinges on finding a residual uninfarcted component. A reticulin stain is helpful for unmasking the architecture of infarcted tissue or, as noted above, immunostains may be useful. Rarely, the diagnosis of a totally infarcted lesion remains enigmatic (Fig. 2.7). If a papillary structure can be demonstrated in this circumstance, the lesion was probably a papilloma rather than a papillary carcinoma because infarction occurs considerably more often in benign papillary tumors than in papillary carcinomas.
GALACTOCELE
The lesions average about 2 cm in diameter, but galactoceles 5 cm or larger have been described (11). Mammography reveals a circumscribed density, which, in many instances, has a characteristic appearance consisting of a hypodense upper area and a lower region with density close to that of the surrounding tissue (12). The interface tends to remain horizontal as the patient changes position. The two zones consist of lighter lipid-containing components above the water-based constituents of the fluid. Comparable differences in echogenicity are observed on ultrasound examination.
The firm, usually painless, tumor may clinically suggest carcinoma. Necrotic cells and nuclear debris, possibly accompanied by inflammatory cells, are seen in a fine-needle
aspiration specimen (13). Cells with hyperchromatic, atypical-appearing nuclei may be present. In this inflammatory background these cells could be mistaken for carcinoma. Excisional biopsy is diagnostic and provides adequate therapy, if the lesion does not resolve after aspiration of the cyst contents.
aspiration specimen (13). Cells with hyperchromatic, atypical-appearing nuclei may be present. In this inflammatory background these cells could be mistaken for carcinoma. Excisional biopsy is diagnostic and provides adequate therapy, if the lesion does not resolve after aspiration of the cyst contents.
A galactocele is composed of cysts lined by nonpapillary cuboidal epithelium. The cysts contain fluid resembling milk. Inspissated secretion may be present in the form of soft, caseous material. Intact cysts are encompassed by a fibrous wall of varying thickness, with little or no inflammatory reaction. Leakage from a cyst elicits a chronic inflammatory reaction that may be accompanied by fat necrosis.
PLASMA CELL MASTITIS
In the early phase, patients experience the acute onset of mild pain, tenderness, redness, and nipple discharge consisting usually of thick secretion. After the inflammatory symptoms subside, the skin may be edematous over a firm-to-hard mass several centimeters in diameter that remains at the same site. Nipple discharge usually persists, and nipple retraction is observed in the majority of patients. Axillary lymph nodes are often enlarged. Plasma cell mastitis in its acute and mature phases is difficult to distinguish clinically from mammary carcinoma. The radiologic findings may be interpreted as indicative of carcinoma, especially when calcifications are present.
Plasma cell mastitis is a form of periductal mastitis characterized by variable hyperplasia of ductal epithelium and a marked, diffuse, plasma cell infiltrate surrounding ducts as well as lobules (Fig. 2.8). A histiocytic reaction to the desquamated epithelium and lipid material in the ducts is responsible for areas that grossly appear to be xanthomatous and for the comedo-like character of the duct contents. Granulomatous features may be present microscopically, especially in areas of necrosis. Lymphocytes and neutrophils are variably present. Periductal fibrosis and obliterative, intraductal proliferation of granulation tissue are not features of plasma cell mastitis. Hyperplastic epithelial cells, which may appear very atypical, can be mistaken for carcinoma in a needle core biopsy sample, leading to an erroneous diagnosis of comedocarcinoma.
MAMMARY DUCT ECTASIA
The earliest symptom is spontaneous, intermittent, mainly watery, nipple discharge. In more advanced cases, subareolar induration progresses to the formation of a mass. The presence of nipple inversion is generally associated with periductal fibrosis and contracture. In some cases, squamous metaplasia of the terminal lactiferous duct epithelium results in obstruction that contributes to the development of duct ectasia and, eventually, to the formation of lactiferous duct fistulas (14,15). The mammographic abnormalities include microcalcifications, spiculated masses, and lobulated, partially smooth masses. In some instances, the mammographic findings suggest carcinoma (16).
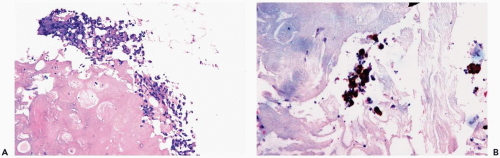
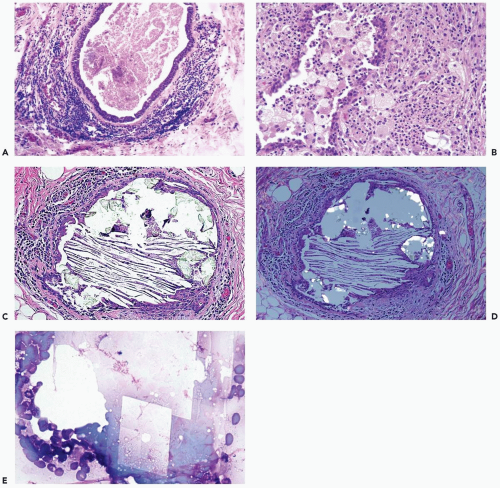
The microscopic composition of the duct contents is variable. The most bland form consists of eosinophilic, granular or amorphous, proteinaceous material. Usually, there is an admixture of lipid-containing histiocytic cells and desquamated duct epithelial cells. Cholesterol crystals and calcifications may be found in the intraductal debris (Fig. 2.9). Histiocytes that contain ceroid pigment, termed ochrocytes by Davies (17), and foam cells may be found within the epithelial-myoepithelial layer of the duct, in periductal tissue, and in the duct lumen (Fig. 2.9). Neutrophils, lymphocytes, and plasma cells within the ducts are usually indicative of a more intense inflammatory reaction (Fig. 2.10). Disruption of ducts is accompanied by discharge of stasis material into the breast, causing periductal inflammation. Plasma cells and granulomas are not
conspicuous features of the lesion, in most cases. Calcium oxalate crystals may be found when stasis occurs in a duct with apocrine epithelium (Fig. 2.10).
conspicuous features of the lesion, in most cases. Calcium oxalate crystals may be found when stasis occurs in a duct with apocrine epithelium (Fig. 2.10).
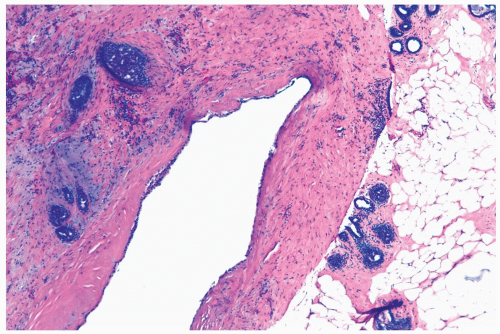 Figure 2.11 Duct ectasia. A late stage lesion sampled by needle core biopsy. There is marked periductal fibrosis with minimal inflammation. |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree