Infective Endocarditis
KEY CONCEPTS
![]() Infective endocarditis is an uncommon infection usually occurring in persons with preexisting cardiac valvular abnormalities (e.g., prosthetic heart valves) or with other specific risk factors (e.g., IV drug abuse).
Infective endocarditis is an uncommon infection usually occurring in persons with preexisting cardiac valvular abnormalities (e.g., prosthetic heart valves) or with other specific risk factors (e.g., IV drug abuse).
![]() Three groups of organisms cause a majority of infective endocarditis cases: streptococci, staphylococci, and enterococci.
Three groups of organisms cause a majority of infective endocarditis cases: streptococci, staphylococci, and enterococci.
![]() The clinical presentation of infective endocarditis is highly variable and nonspecific, although a fever and murmur are usually present. Classic peripheral manifestations (e.g., Osler’s nodes) may or may not occur.
The clinical presentation of infective endocarditis is highly variable and nonspecific, although a fever and murmur are usually present. Classic peripheral manifestations (e.g., Osler’s nodes) may or may not occur.
![]() The diagnosis of infective endocarditis requires the integration of clinical, laboratory, and echocardiographic findings. The two major diagnostic criteria are bacteremia and echocardiographic changes (e.g., valvular vegetation).
The diagnosis of infective endocarditis requires the integration of clinical, laboratory, and echocardiographic findings. The two major diagnostic criteria are bacteremia and echocardiographic changes (e.g., valvular vegetation).
![]() Treatment of infective endocarditis involves isolation of the infecting pathogen and determination of antimicrobial susceptibilities, followed by high-dose, parenteral, bactericidal antibiotics for an extended period.
Treatment of infective endocarditis involves isolation of the infecting pathogen and determination of antimicrobial susceptibilities, followed by high-dose, parenteral, bactericidal antibiotics for an extended period.
![]() Surgical replacement of the infected heart valve is an important adjunct to endocarditis treatment in certain situations (e.g., patients with acute heart failure).
Surgical replacement of the infected heart valve is an important adjunct to endocarditis treatment in certain situations (e.g., patients with acute heart failure).
![]() β-Lactam antibiotics, such as penicillin G (or ceftriaxone), nafcillin, and ampicillin, remain the drugs of choice for streptococcal, staphylococcal, and enterococcal endocarditis, respectively.
β-Lactam antibiotics, such as penicillin G (or ceftriaxone), nafcillin, and ampicillin, remain the drugs of choice for streptococcal, staphylococcal, and enterococcal endocarditis, respectively.
![]() Aminoglycoside antibiotics are essential to obtain a synergistic bactericidal effect in the treatment of enterococcal endocarditis. Adjunctive aminoglycosides also may decrease the emergence of resistant organisms (e.g., prosthetic valve endocarditis caused by coagulase-negative staphylococci) and hasten the pace of clinical and microbiologic response (e.g., some streptococcal and staphylococcal infections).
Aminoglycoside antibiotics are essential to obtain a synergistic bactericidal effect in the treatment of enterococcal endocarditis. Adjunctive aminoglycosides also may decrease the emergence of resistant organisms (e.g., prosthetic valve endocarditis caused by coagulase-negative staphylococci) and hasten the pace of clinical and microbiologic response (e.g., some streptococcal and staphylococcal infections).
![]() Vancomycin is reserved for patients with immediate β-lactam allergies and the treatment of resistant organisms.
Vancomycin is reserved for patients with immediate β-lactam allergies and the treatment of resistant organisms.
![]() Antimicrobial prophylaxis is used as an attempt to prevent infective endocarditis for patients who are at the highest risk (such as persons with prosthetic heart valves) before a bacteremia-causing procedure (e.g., dental extraction).
Antimicrobial prophylaxis is used as an attempt to prevent infective endocarditis for patients who are at the highest risk (such as persons with prosthetic heart valves) before a bacteremia-causing procedure (e.g., dental extraction).
Endocarditis is an inflammation of the endocardium, the membrane lining the chambers of the heart and covering the cusps of the heart valves.1,2 More commonly, endocarditis refers to infection of the heart valves by various microorganisms. Although it typically affects native valves, it also may involve nonvalvular areas or implanted mechanical devices (e.g., mechanical heart valves). Bacteria primarily cause endocarditis, but fungi and other atypical microorganisms can lead to the disease; hence, the more encompassing term infective endocarditis is preferred.2,3
Endocarditis is often referred to as acute or subacute depending on the pace and severity of the clinical presentation. The acute, fulminating form is associated with high fevers and systemic toxicity. Virulent bacteria, such as Staphylococcus aureus, frequently cause this syndrome, and if untreated, death may occur within days to weeks. On the other hand, subacute infective endocarditis is more indolent, and it is caused by less invasive organisms, such as viridans streptococci, usually occurring in preexisting valvular heart disease. Although infective endocarditis is often referred to as acute or subacute, it is best classified based on the etiologic organism, the anatomic site of infection, and pathogenic risk factors.1,4,5 Infection also may follow surgical insertion of a prosthetic heart valve, resulting in prosthetic valve endocarditis (PVE), or insertion of a cardiac implantable electronic device, resulting in cardiac device infective endocarditis (CDIE).6,7
EPIDEMIOLOGY AND ETIOLOGY
Infective endocarditis is an uncommon, but not rare, infection. Population-based studies have reported incidence rates of 2 to 15 cases per 100,000 person-years.1,8,9 In the United States, the infection is listed as the primary or secondary diagnosis of 28,000 hospital discharges.10 The mean male-to-female ratio is approximately 2:1.11 As the population ages and as valve replacement surgery becomes more common, the mean age of patients with infective endocarditis increases. Overall, most cases occur in individuals older than 50 years of age, and it is uncommon in children.11–14 PVE and CDIE account for 20% and 6.4% of cases of infective endocarditis, respectively.11,15 Those with a history of IV drug abuse (IVDA) are also at high risk. Of note, the incidence of healthcare-associated infective endocarditis is rising, especially in the elderly population.11,12 Other conditions associated with a higher incidence of infective endocarditis include diabetes, long-term hemodialysis, and poor dental hygiene.5
![]() Most persons with infective endocarditis have risk factors, such as preexisting cardiac valvular abnormalities. Many types of structural heart disease result in turbulent blood flow that increases the risk for infective endocarditis. A predisposing risk factor, however, may be absent in up to 25% of cases. Some of the more important risk factors include5,7,11,13,16:
Most persons with infective endocarditis have risk factors, such as preexisting cardiac valvular abnormalities. Many types of structural heart disease result in turbulent blood flow that increases the risk for infective endocarditis. A predisposing risk factor, however, may be absent in up to 25% of cases. Some of the more important risk factors include5,7,11,13,16:
1. Presence of a prosthetic valve (highest risk)
2. Previous endocarditis (highest risk)
3. Congenital heart disease (CHD)
4. Chronic IV access
5. Diabetes mellitus
6. Healthcare-related exposure
7. Acquired valvular dysfunction (e.g., rheumatic heart disease)
8. Cardiac implantable device
9. Chronic heart failure
10. Mitral valve prolapse with regurgitation
11. IVDA
In the past, rheumatic heart disease was a prevalent risk factor for infective endocarditis, but the incidence of this disease continues to decline. The risk of infective endocarditis in persons with mitral valve prolapse and regurgitation is small; however, because the condition is prevalent, it is an important contributor to the overall number of infective endocarditis cases.5,11 PVE occurs in 1% to 3% of patients undergoing valve replacement surgery in the first postoperative year.11,17
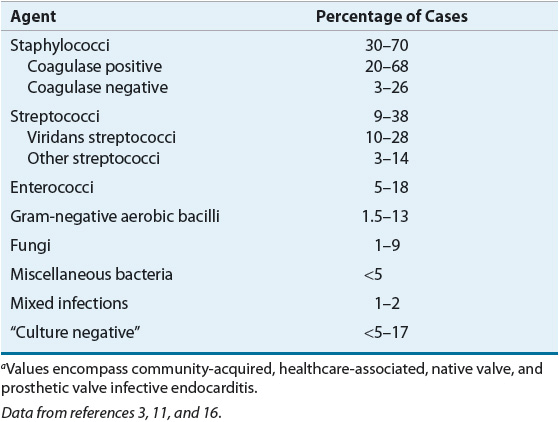
![]() Nearly every organism causing human disease may cause infective endocarditis, but three groups of organisms result in a majority of cases: streptococci, staphylococci, and enterococci (Table 89–1).1,3,4,11,16 The incidence of staphylococci, particularly S. aureus, continues to increase, and case series have documented that staphylococci have surpassed viridans streptococci as the leading cause of infective endocarditis.11,16 In general, streptococci cause infective endocarditis in patients with community-acquired disease and underlying cardiac abnormalities, such as mitral valve prolapse or rheumatic heart disease. Staphylococci (S. aureus and coagulase-negative staphylococci) are the most common cause of PVE within the first year after valve surgery, and S. aureus is common in those with a history of IVDA. Although polymicrobial infective endocarditis is uncommon, it is encountered most often in association with IVDA.1,11,16 Enterococcal endocarditis tends to follow genitourinary manipulations or obstetric procedures.17 There are many exceptions to the preceding generalizations; thus, isolation of the causative pathogen and determination of its antimicrobial susceptibilities offer the best chance for successful therapy.
Nearly every organism causing human disease may cause infective endocarditis, but three groups of organisms result in a majority of cases: streptococci, staphylococci, and enterococci (Table 89–1).1,3,4,11,16 The incidence of staphylococci, particularly S. aureus, continues to increase, and case series have documented that staphylococci have surpassed viridans streptococci as the leading cause of infective endocarditis.11,16 In general, streptococci cause infective endocarditis in patients with community-acquired disease and underlying cardiac abnormalities, such as mitral valve prolapse or rheumatic heart disease. Staphylococci (S. aureus and coagulase-negative staphylococci) are the most common cause of PVE within the first year after valve surgery, and S. aureus is common in those with a history of IVDA. Although polymicrobial infective endocarditis is uncommon, it is encountered most often in association with IVDA.1,11,16 Enterococcal endocarditis tends to follow genitourinary manipulations or obstetric procedures.17 There are many exceptions to the preceding generalizations; thus, isolation of the causative pathogen and determination of its antimicrobial susceptibilities offer the best chance for successful therapy.
TABLE 89-1 Etiologic Organisms in Infective Endocarditisa
The mitral and aortic valves are affected most commonly in cases involving a single valve. Subacute endocarditis tends to involve the mitral valve, whereas acute disease often involves the aortic valve. Up to 35% of cases involve concomitant infections of both the aortic and the mitral valves. Infection of the tricuspid valve is less common, with a majority of these cases occurring in patients with a history of IVDA. It is rare for the pulmonary valve to be infected.11,16,17
Pathophysiology
The development of infective endocarditis via hematogenous spread, the most common route, requires the sequential occurrence of several factors. These components are complex and not fully elucidated.18,19
1. The endothelial surface of the heart is damaged. This injury occurs with turbulent blood flow associated with the valvular lesions previously described.
2. Platelet and fibrin deposition occurs on the abnormal epithelial surface. These platelet–fibrin deposits are referred to as nonbacterial thrombotic endocarditis.
3. Bacteremia gives organisms access to and results in colonization of the endocardial surface. Bacteremia is the result of trauma to a mucosal surface with a high concentration of resident bacteria, such as the oral cavity and GI tract. Transient bacteremia commonly follow certain dental, GI, urologic, and gynecologic procedures. Staphylococci, viridans streptococci, and enterococci are most likely to adhere to nonbacterial thrombotic endocarditis, probably because of production of specific adherence factors, such as dextran by some oral streptococci and glycocalyx for staphylococci. Gram-negative bacteria rarely adhere to heart valves and are uncommon causes of infective endocarditis.
4. After colonization of the endothelial surface, a “vegetation” of fibrin, platelets, and bacteria forms. The protective cover of fibrin and platelets allows unimpeded bacterial growth to concentrations as high as 109 to 1010 organisms per gram of tissue.
The pathogenesis of early PVE or CDIE differs from infective endocarditis acquired by the hematogenous route because surgery may directly inoculate prosthetic material with bacteria from the patient’s skin or operating room personnel. In the case of early PVE, a recently placed nonendothelialized valve is more susceptible to bacterial colonization than are native valves. Bacteria also may colonize the new valve from contaminated bypass pumps, cannulas, and pacemakers or from a nosocomial bacteremia subsequent to an intravascular catheter.7,16,17 The mechanism of bacterial colonization and pathogenesis in late PVE is similar to native valve endocarditis (NVE).17
The vegetations seen in infective endocarditis may be single or multiple and vary in size from a few millimeters to centimeters. Bacteria within the vegetation grow slowly and are protected from antibiotics and host defenses. The adverse effects of infective endocarditis and the resulting lesions can be far-reaching and include (a) local perivalvular damage, (b) embolization of septic fragments with potential hematogenous seeding of remote sites, and (c) formation of antibody complexes.17,19
Formation of vegetations may destroy valvular tissue, and continued destruction can lead to acute heart failure via perforation of the valve leaflet, rupture of the chordae tendineae or papillary muscle, or, for patients with PVE, valve dehiscence. Occasionally, valvular stenosis may occur. Abscesses can develop in the valve ring or in myocardial tissue itself. Even with resolution of the process, fibrosis of tissue with some residual dysfunction is possible.
Vegetations may be friable, and fragments may be released downstream. These infected particles, termed septic emboli, can result in organ abscess or infarction. Septic emboli from right-sided endocarditis commonly lodge in the lungs, causing pulmonary abscesses. Emboli from left-sided vegetations commonly affect organs with high blood flow, such as the kidneys, spleen, and brain.17,19
Circulating immune complexes consisting of antigen, antibody, and complement may deposit in organs, producing local inflammation and damage (e.g., glomerulonephritis in the kidneys). Other potential pathologic changes that result from immune-complex deposition or septic emboli include the development of “mycotic” aneurysms (although the aneurysm is usually bacterial in origin, not fungal), cerebral infarction, splenic infarction and abscess, and skin manifestations such as petechiae, Osler’s nodes, and Janeway’s lesions.5,17,19
CLINICAL PRESENTATION
![]() The clinical presentation of infective endocarditis is highly variable and nonspecific. Fever is the most common finding and is often accompanied by other vague symptoms (Table 89–2). Fever may be relatively low grade, particularly in subacute cases. Heart murmurs are found in a majority of patients, most often preexisting, with some documented as new or changing. Infective endocarditis usually begins insidiously and worsens gradually. Patients may present with nonspecific findings, such as fever, chills, weakness, dyspnea, night sweats, weight loss, or malaise. In contrast, patients with acute disease, such as those with a history of IVDA and S. aureus infective endocarditis, may appear with classic signs of sepsis.
The clinical presentation of infective endocarditis is highly variable and nonspecific. Fever is the most common finding and is often accompanied by other vague symptoms (Table 89–2). Fever may be relatively low grade, particularly in subacute cases. Heart murmurs are found in a majority of patients, most often preexisting, with some documented as new or changing. Infective endocarditis usually begins insidiously and worsens gradually. Patients may present with nonspecific findings, such as fever, chills, weakness, dyspnea, night sweats, weight loss, or malaise. In contrast, patients with acute disease, such as those with a history of IVDA and S. aureus infective endocarditis, may appear with classic signs of sepsis.
TABLE 89-2 Clinical Presentation of Infective Endocarditis

Splenomegaly is a frequent finding for patients with prolonged endocarditis. Other important clinical signs especially prevalent in subacute illness may include the following peripheral manifestations (“stigmata”) of endocarditis5,11,12,16:
1. Osler’s nodes: Purplish or erythematous subcutaneous papules or nodules on the pads of the fingers and toes. These lesions are 2 to 15 mm in size and are painful and tender. These nodes are not specific for infective endocarditis and may be the result of embolism, immunologic phenomena, or both.
2. Janeway’s lesions: Hemorrhagic, painless plaques on the palms of the hands or soles of the feet. These lesions are believed to be embolic in origin.
3. Splinter hemorrhages: Thin, linear hemorrhages found under the nail beds of the fingers or toes. These lesions are not specific for infective endocarditis and more commonly are the result of traumatic injuries. Distal lesions are more likely the result of trauma, whereas proximal lesions tend to be associated with infective endocarditis.
4. Petechiae: Small (usually 1 to 2 mm in diameter), erythematous, painless, hemorrhagic lesions. These lesions appear anywhere on the skin but more frequently on the anterior trunk, buccal mucosa and palate, and conjunctivae. Petechiae are nonblanching and resolve after a few days.
5. Clubbing of the fingers: Proliferative changes in the soft tissues about the terminal phalanges observed in long-standing endocarditis.
6. Roth’s spots: Retinal infarct with central pallor and surrounding hemorrhage.
7. Emboli: Embolic phenomena occur in up to one third of cases and may result in significant complications. Left-sided endocarditis can result in renal artery emboli causing flank pain with hematuria, splenic artery emboli causing abdominal pain, and cerebral emboli, which may result in hemiplegia or alteration in mental status. Right-sided endocarditis may result in pulmonary emboli, causing pleuritic pain with hemoptysis.
Patients with infective endocarditis typically have laboratory abnormalities; however, none of these changes is specific for the disease. Anemia (normocytic, normochromic), leukocytosis, and thrombocytopenia may be present. The white blood cell count is often normal or only slightly elevated, sometimes with a mild left shift. Acute bacterial endocarditis, however, may present with an elevated white blood cell count, consistent with a fulminant infection. The erythrocyte sedimentation rate and C-reactive protein may be elevated in approximately 60% of patients. Often the urinary analysis is abnormal, with proteinuria and microscopic hematuria occurring in approximately 25% of individuals.5,11
The hallmark of infective endocarditis is a continuous bacteremia caused by bacteria shedding from the vegetation into the bloodstream; 90% to 95% of patients with infective endocarditis have positive blood cultures.1,11,17 Three sets of blood cultures, each from separate venipuncture sites, should be collected over 24 hours, and antibiotics should be withheld until adequate blood cultures are obtained. On the other hand, if a patient has a toxic appearance, several blood cultures should be collected promptly, followed by immediate empirical antimicrobial treatment. The blood cultures for patients who have received previous antibiotics should be monitored more closely because pathogen growth may be suppressed.1 “Culture-negative” endocarditis describes a patient in whom a clinical diagnosis of infective endocarditis is likely but blood cultures do not yield a pathogen. This condition is often the consequence of previous antibiotic therapy, improperly collected blood cultures, or unusual organisms.4 When blood cultures from patients suspected of having infective endocarditis show no growth after 48 to 72 hours, the laboratory should be advised and cultures held for up to a month to detect growth of fastidious organisms.4
An electrocardiogram, chest radiograph, and echocardiogram are performed for patients suspected of endocarditis. The electrocardiogram rarely shows important diagnostic findings but may reveal heart block, suggesting extension of the infection. The chest radiograph may provide more diagnostic information, especially in a patient with right-sided endocarditis. Septic pulmonary emboli may occur, leading to multiple lung foci. The echocardiogram is the most important test and should be performed for all patients suspected of this infection.
Echocardiography plays an important role in the diagnosis and management of infective endocarditis.4 The chosen approach, transthoracic echocardiography (TTE) or transesophageal echocardiography (TEE), depends on the clinical setting. The TEE technique is more sensitive for detecting vegetations (90% to 100%) as compared with TTE (58% to 63%), and TEE maintains good specificity (85% to 95%).1,4 TTE appears reasonable in the evaluation of children or adults in whom the clinical suspicion of infective endocarditis is relatively low.4,20 TEE is preferred in high-risk patients such as those with prosthetic heart valves, many CHDs, previous endocarditis, new murmur, heart failure, or other stigmata of endocarditis.4,21,22 The lack of vegetation on echocardiogram does not exclude infection even if the transesophageal approach is used. Conversely, the test may reveal an unsuspected large vegetation, extension of the disease into surrounding tissue, valvular defects, abscess formation, cordial rupture, or an intracardiac fistula. Thus, in addition to helping in the diagnosis of infective endocarditis, the echocardiogram allows the physician to evaluate hemodynamic stability and the need for urgent surgical intervention; it also provides a rough estimate of the likelihood of embolism.4,22
DIAGNOSIS
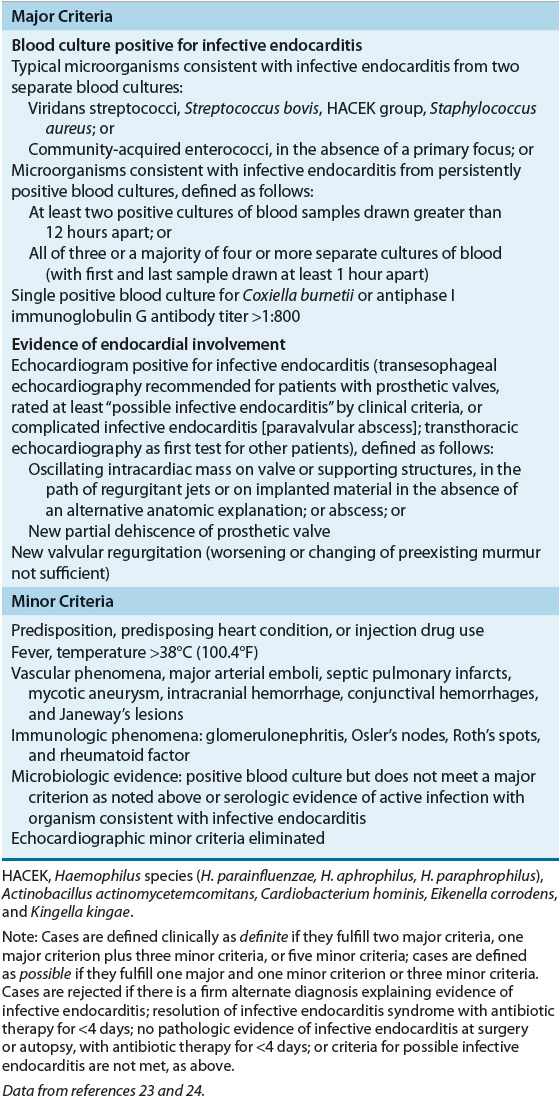
![]() The signs and symptoms of infective endocarditis are not specific, and the diagnosis is often unclear. The identification of infective endocarditis requires the integration of clinical, laboratory, and echocardiographic findings. The Duke diagnostic criteria include major and minor variables (Table 89–3).23,24 Based on the number of major and minor criteria that are fulfilled, patients suspected of infective endocarditis are categorized into three separate groups: definite infective endocarditis, possible infective endocarditis, or infective endocarditis rejected.24
The signs and symptoms of infective endocarditis are not specific, and the diagnosis is often unclear. The identification of infective endocarditis requires the integration of clinical, laboratory, and echocardiographic findings. The Duke diagnostic criteria include major and minor variables (Table 89–3).23,24 Based on the number of major and minor criteria that are fulfilled, patients suspected of infective endocarditis are categorized into three separate groups: definite infective endocarditis, possible infective endocarditis, or infective endocarditis rejected.24
TABLE 89-3 Diagnosis of Infective Endocarditis According to the Modified Duke Criteria
PROGNOSIS
The outcome for endocarditis is improved with rapid diagnosis, appropriate treatment (i.e., antimicrobial therapy, surgery, or both), and prompt recognition of complications should they arise. Factors associated with increased mortality include (a) heart failure, (b) increasing age, (c) endocarditis caused by resistant organisms such as fungi or gram-negative bacteria, (d) left-sided endocarditis caused by S. aureus, (e) paravalvular complications, (f) healthcare-acquired infection, and (g) PVE.4,5,11,16 The presence of heart failure has the greatest negative impact on the short-term prognosis.4 For left-sided native valve infective endocarditis, mortality rates range from 15% to 45%; lower rates (4% to 16%) occur with community-acquired disease that is most commonly caused by viridans streptococci. Higher rates (25% to 45%) occur with healthcare-associated disease that is more commonly caused by enterococci and staphylococci.1 Even higher rates of mortality are seen with unusually encountered organisms (e.g., mortality greater than 50% for Pseudomonas aeruginosa).4 The mortality rate for right-sided infective endocarditis associated with IVDA is generally low (e.g., less than 10%).1,4 For those who relapse after treatment for infective endocarditis, most will do so within the first 2 months after discontinuation of antimicrobials. Relapse rates for viridans streptococcus are generally low (2%), whereas relapse is more likely in those with enterococcal infection (8% to 20%) and PVE (10% to 15%).5,17 After appropriate treatment and recovery, the risk of morbidity and mortality following infective endocarditis persists for years, although it gradually declines annually. Morbidity remains elevated because of a greater likelihood of recurrent infective endocarditis, heart failure, and embolism or, if a valve is replaced, the risk of anticoagulation, valve thrombosis, or additional valve surgery.5,21
TREATMENT
Desired Outcomes
The desired outcomes for treatment and prophylaxis of infective endocarditis are to:
1. Relieve the signs and symptoms of the disease
2. Decrease morbidity and mortality associated with the infection
3. Eradicate the causative organism with minimal drug exposure
4. Provide cost-effective antimicrobial therapy determined by the likely or identified pathogen, drug susceptibilities, hepatic and renal function, drug allergies, and anticipated drug toxicities
5. Prevent infective endocarditis from occurring or recurring in high-risk patients with appropriate prophylactic antimicrobials
General Approach to Treatment
![]() The most important approach in the treatment of infective endocarditis is isolation of the infecting pathogen and determination of antimicrobial susceptibilities, followed by high-dose, parenteral, bactericidal antibiotics for an extended period.1,5,17 Identification of susceptibilities is crucial given the escalating level of antibiotic resistance to commonly encountered pathogens. Treatment usually is started in the hospital, but for select patients it is often completed in the outpatient setting so long as defervescence has occurred and followup blood cultures show no growth.5,25 Large doses of parenteral antimicrobials usually are necessary to achieve bactericidal concentrations within vegetations. An extended duration of therapy is required, even for susceptible pathogens, because microorganisms are enclosed within valvular vegetations and fibrin deposits. These barriers impair host defenses and protect microbes from phagocytic cells. In addition, high bacterial concentrations within vegetations may result in an inoculum effect that further resists killing (see eChap. 24 for additional discussion). Many bacteria are not actively dividing, further limiting the rate of bacterial death. For most patients, a minimum of 4 to 6 weeks of therapy is required.4
The most important approach in the treatment of infective endocarditis is isolation of the infecting pathogen and determination of antimicrobial susceptibilities, followed by high-dose, parenteral, bactericidal antibiotics for an extended period.1,5,17 Identification of susceptibilities is crucial given the escalating level of antibiotic resistance to commonly encountered pathogens. Treatment usually is started in the hospital, but for select patients it is often completed in the outpatient setting so long as defervescence has occurred and followup blood cultures show no growth.5,25 Large doses of parenteral antimicrobials usually are necessary to achieve bactericidal concentrations within vegetations. An extended duration of therapy is required, even for susceptible pathogens, because microorganisms are enclosed within valvular vegetations and fibrin deposits. These barriers impair host defenses and protect microbes from phagocytic cells. In addition, high bacterial concentrations within vegetations may result in an inoculum effect that further resists killing (see eChap. 24 for additional discussion). Many bacteria are not actively dividing, further limiting the rate of bacterial death. For most patients, a minimum of 4 to 6 weeks of therapy is required.4
Nonpharmacologic Therapy
![]() Surgery is an important adjunct in the management of both NVE and PVE. In most surgical cases, valvectomy and valve replacement are performed to remove infected tissue and to restore hemodynamic function. Indications for surgery include heart failure, persistent bacteremia, persistent vegetation, an increase in vegetation size, or recurrent emboli despite prolonged antibiotic treatment, valve dysfunction, paravalvular extension (e.g., abscess), or endocarditis caused by resistant organisms (e.g., fungi or gram-negative bacteria).4,26,27 More controversial is the appropriate timing of surgery as American and European guidelines have different criteria for emergent or urgent surgical intervention.26,27 Additionally, studies evaluating postsurgical outcomes and associated mortality are limited such that a specific risk prediction system has not been established.28–32 Early surgery (e.g., within 48 hours) may be appropriate in patients with severe heart failure and large vegetations, whereas patients with septic shock, advanced age, or neurologic complications of infective endocarditis may have more detrimental outcomes.28,29,33,34
Surgery is an important adjunct in the management of both NVE and PVE. In most surgical cases, valvectomy and valve replacement are performed to remove infected tissue and to restore hemodynamic function. Indications for surgery include heart failure, persistent bacteremia, persistent vegetation, an increase in vegetation size, or recurrent emboli despite prolonged antibiotic treatment, valve dysfunction, paravalvular extension (e.g., abscess), or endocarditis caused by resistant organisms (e.g., fungi or gram-negative bacteria).4,26,27 More controversial is the appropriate timing of surgery as American and European guidelines have different criteria for emergent or urgent surgical intervention.26,27 Additionally, studies evaluating postsurgical outcomes and associated mortality are limited such that a specific risk prediction system has not been established.28–32 Early surgery (e.g., within 48 hours) may be appropriate in patients with severe heart failure and large vegetations, whereas patients with septic shock, advanced age, or neurologic complications of infective endocarditis may have more detrimental outcomes.28,29,33,34
Clinical Controversy…
Pharmacologic Therapy
Specific treatment recommendations from the American Heart Association (AHA) provide guidance for the management of infective endocarditis, and these were updated in 2005.4 Guidelines published in 2009 by the European Society of Cardiology are consistent with the AHA guidelines.27 Both guidelines use an evidence-based scoring system where recommendations are given a classification as well as level of evidence. Class I recommendations are conditions for which there is evidence, general agreement, or both that a given procedure or treatment is useful and effective. Class II recommendations are conditions for which there is conflicting evidence, a divergence of opinion, or both about the usefulness/efficacy of a procedure or treatment (IIa implies the weight of evidence/opinion is in favor of usefulness/efficacy, whereas IIb implies usefulness/efficacy is less well established by evidence/opinion). Class III recommendations are conditions for which there is evidence, general agreement, or both that the procedure/treatment is not useful/effective and in some cases may be harmful. Level of evidence is listed as A (data derived from multiple randomized clinical trials), B (data derived from a single randomized trial or nonrandomized studies), and C (consensus opinion of experts).
![]() β-Lactam antibiotics, such as penicillin G (or ceftriaxone), nafcillin, and ampicillin, remain the drugs of choice for streptococcal, staphylococcal, and enterococcal endocarditis, respectively. Tables 89–4 to 89-7 summarize these recommendations, which are discussed in more detail in the following sections. Tables 89–8 and 89-9 list drug dosing and monitoring recommendations for adult and pediatric patients. Because these guidelines focus on common causes of endocarditis, readers are referred to other references for more in-depth discussion of unusually encountered organisms.4,27,35–37
β-Lactam antibiotics, such as penicillin G (or ceftriaxone), nafcillin, and ampicillin, remain the drugs of choice for streptococcal, staphylococcal, and enterococcal endocarditis, respectively. Tables 89–4 to 89-7 summarize these recommendations, which are discussed in more detail in the following sections. Tables 89–8 and 89-9 list drug dosing and monitoring recommendations for adult and pediatric patients. Because these guidelines focus on common causes of endocarditis, readers are referred to other references for more in-depth discussion of unusually encountered organisms.4,27,35–37
TABLE 89-4 Treatment Options for Native Valve Endocarditis by Causative Organism
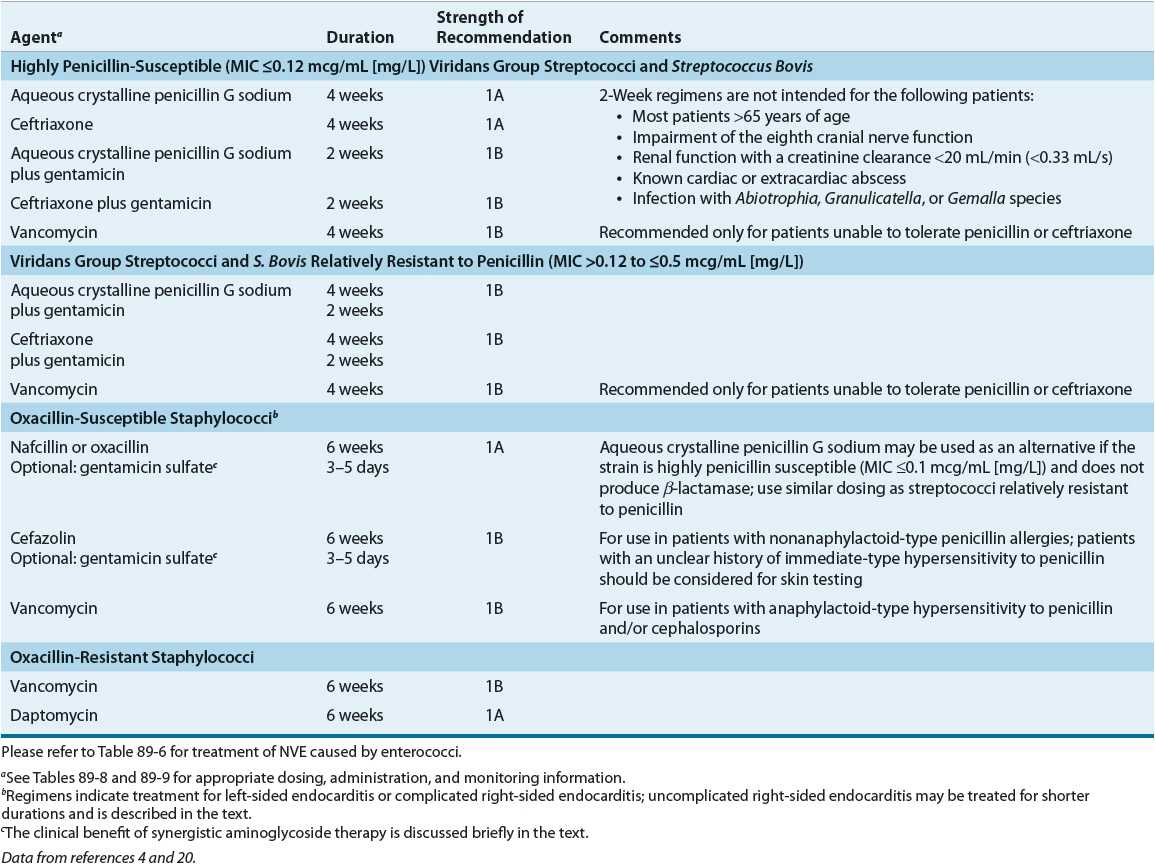
TABLE 89-5 Treatment Options for Prosthetic Valve Endocarditis (PVE) by Causative Organism
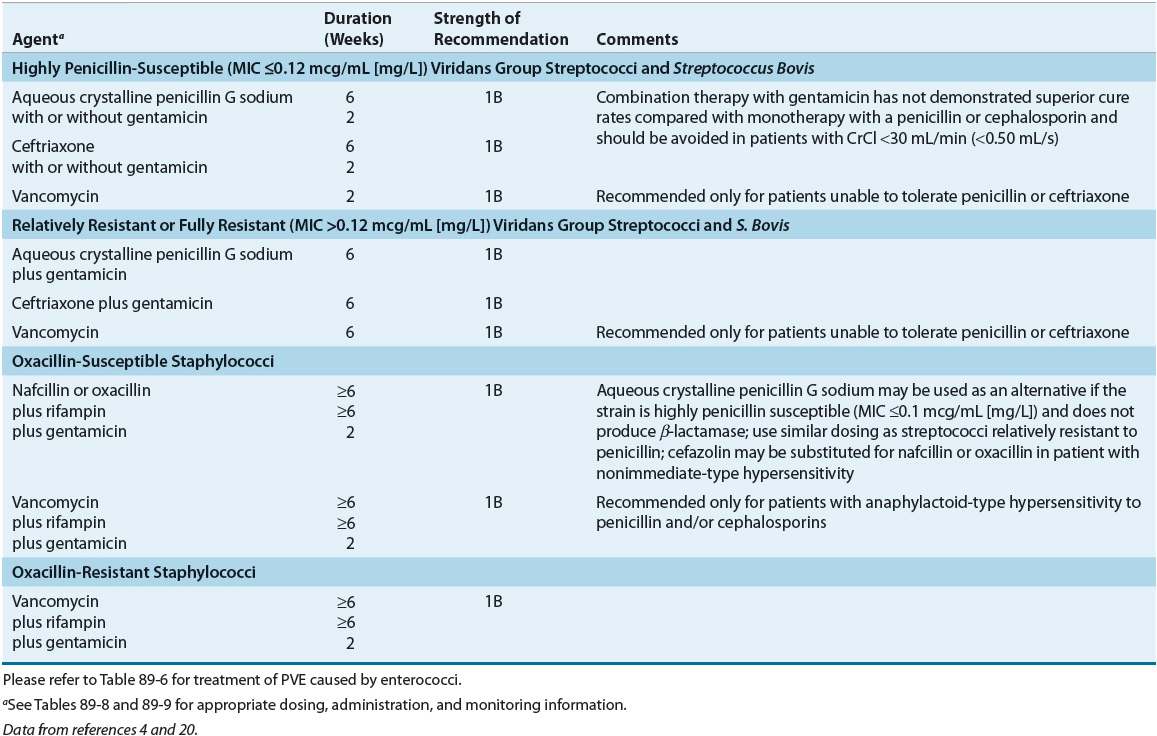
TABLE 89-6 Treatment Options for Native or Prosthetic Valve Endocarditis Caused by Enterococci
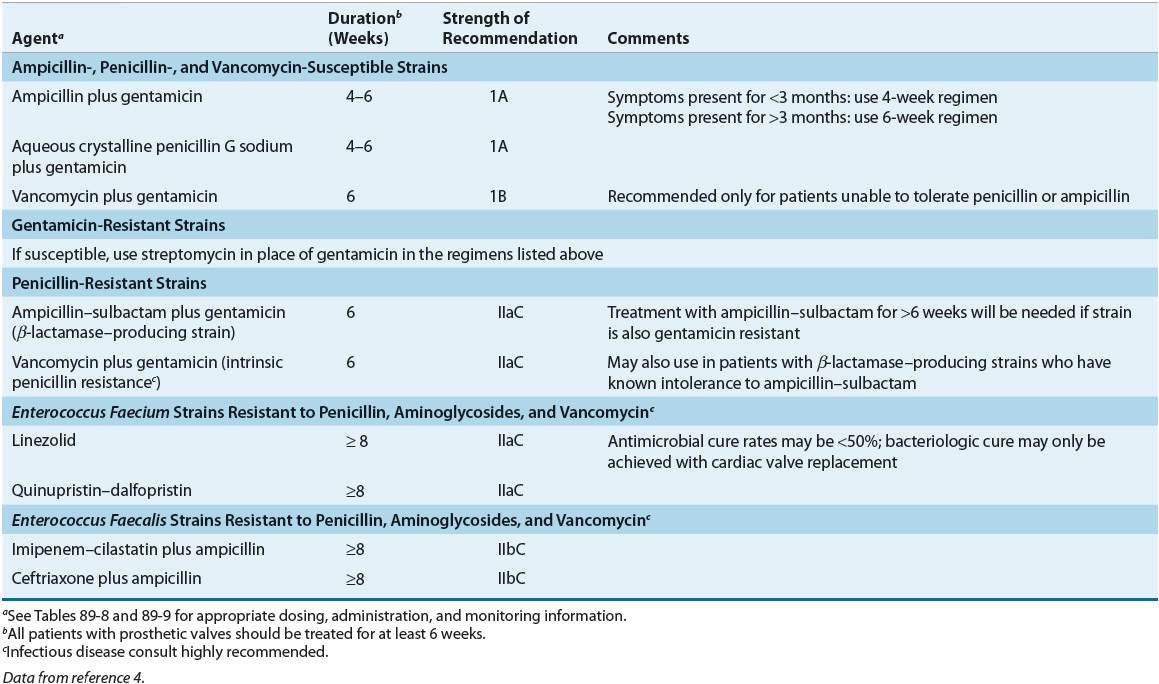
TABLE 89-7 Treatment Options for Culture-Negative Endocarditis and Endocarditis Caused by Gram-Negative Organismsa
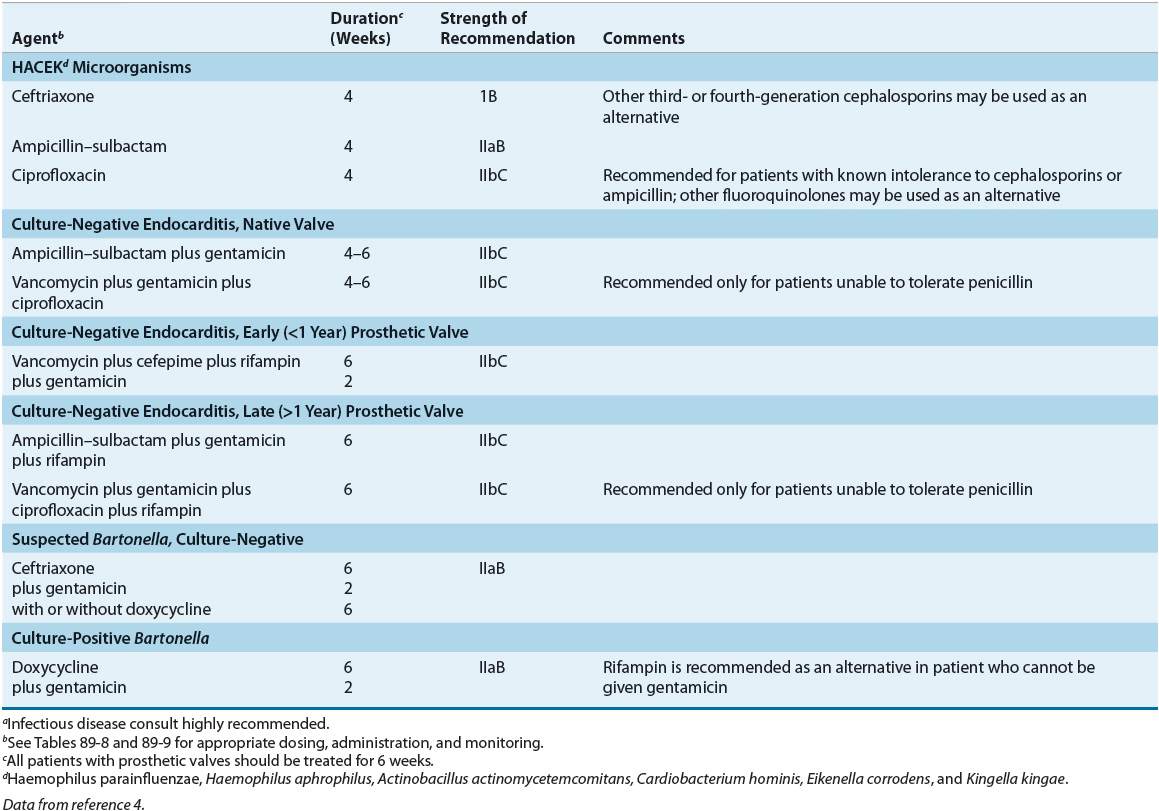
TABLE 89-8 Drug Dosing Table for Treatment of Infective Endocarditisa
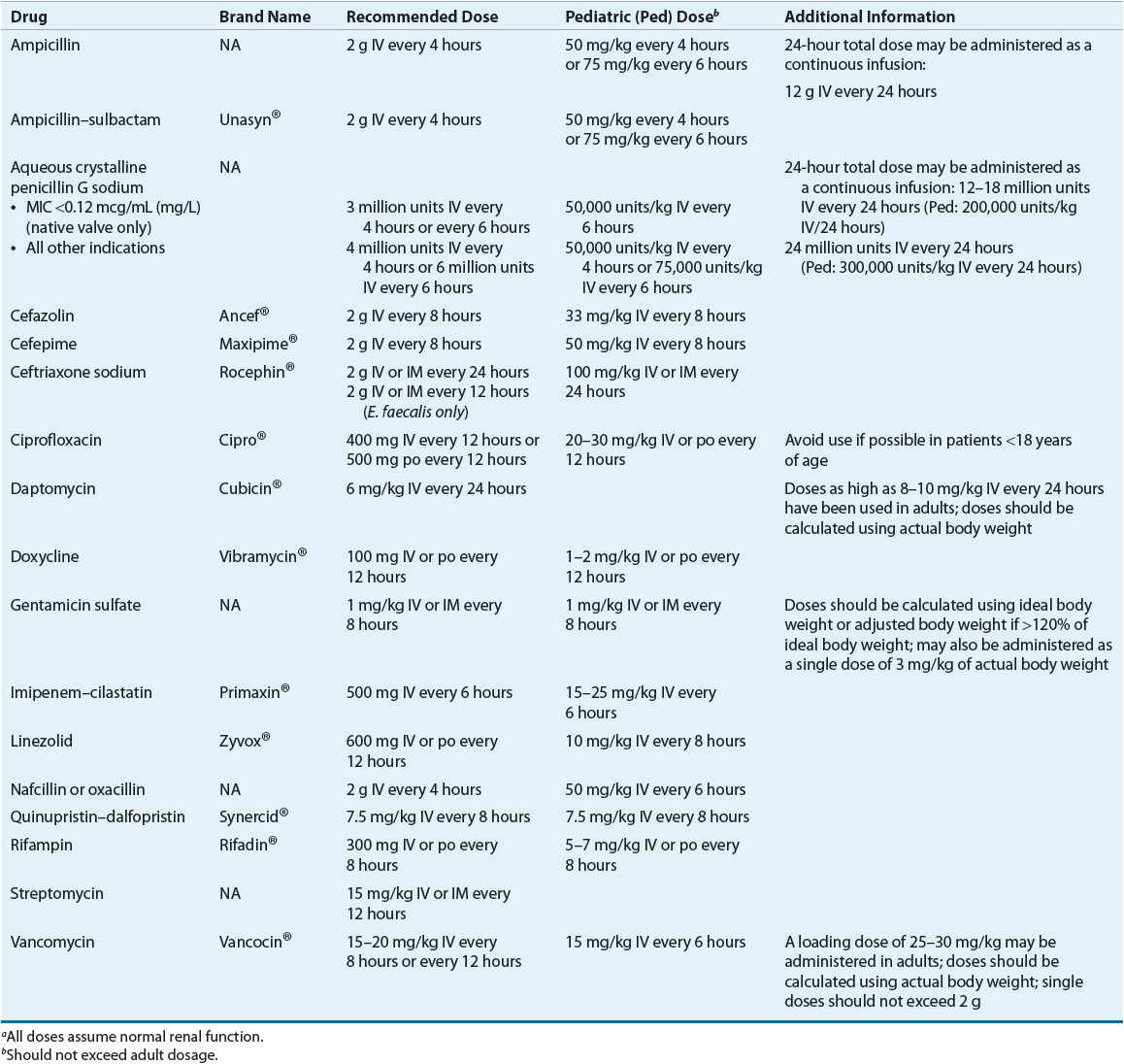
TABLE 89-9 Drug Monitoring of Select Agents
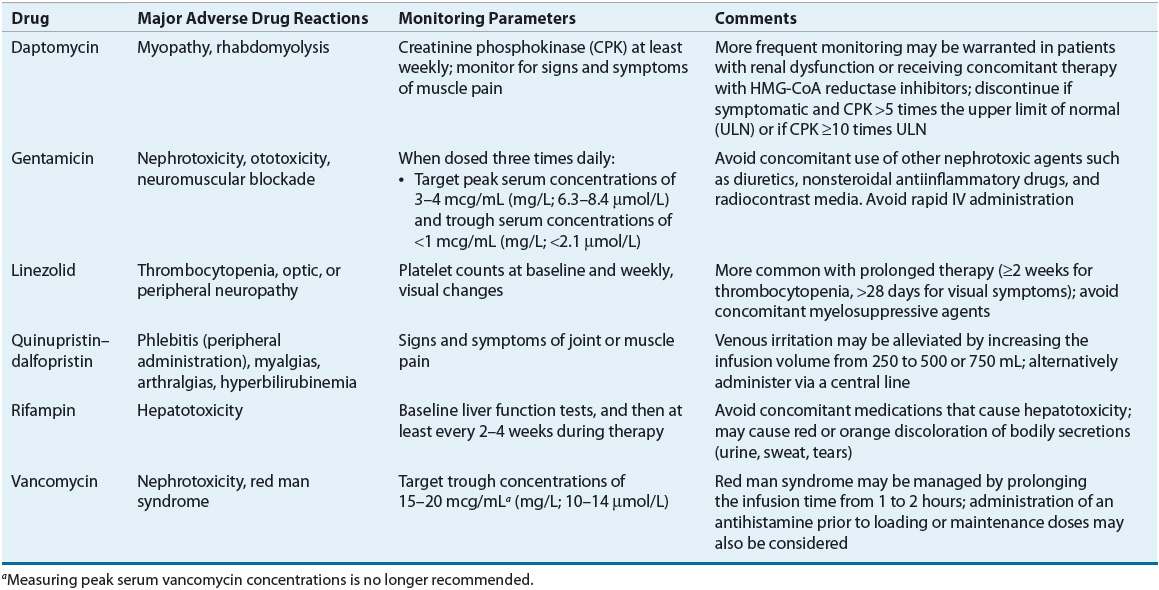
![]() For some pathogens, such as enterococci, the use of synergistic antimicrobial combinations (including an aminoglycoside) is essential to obtain a bactericidal effect. Combination antibiotics also may decrease the emergence of resistant organisms during treatment (e.g., PVE caused by coagulase-negative staphylococci) and hasten the pace of clinical and microbiologic response (e.g., some streptococcal and staphylococcal infections). Occasionally, combination treatment will result in a shorter treatment course.
For some pathogens, such as enterococci, the use of synergistic antimicrobial combinations (including an aminoglycoside) is essential to obtain a bactericidal effect. Combination antibiotics also may decrease the emergence of resistant organisms during treatment (e.g., PVE caused by coagulase-negative staphylococci) and hasten the pace of clinical and microbiologic response (e.g., some streptococcal and staphylococcal infections). Occasionally, combination treatment will result in a shorter treatment course.
Streptococcal Endocarditis
Streptococci are a common cause of infective endocarditis, with most isolates being viridans streptococci. Viridans streptococci refer to a large number of different species, such as Streptococcus sanguinis, Streptococcus oralis, Streptococcus salivarius, Streptococcus mutans, and Gemella morbillorum.4 These bacteria are common inhabitants of the human mouth and gingiva, and they are especially common causes of endocarditis involving native valves.1,4,16 During dental surgery, and even when brushing the teeth, these organisms can cause a transient bacteremia. In susceptible individuals, this may result in infective endocarditis. Streptococcal endocarditis is usually subacute, and the response to medical treatment is very good. Streptococcus bovis is not a viridans streptococcus, but it is included in this treatment group because it is penicillin sensitive and requires the same treatment as viridans streptococci. S. bovis is a nonenterococcal group D Streptococcus that resides in the GI tract. Infective endocarditis caused by this organism is often associated with a GI pathology, especially colon carcinoma. Endocarditis caused by Streptococcus pneumoniae, Streptococcus pyogenes, and group B, C, and G streptococci are uncommon, and their treatment is not well defined.4,19
Antimicrobial regimens for viridans streptococci are well studied, and in uncomplicated cases, response rates as high as 98% can be expected. Viridans streptococci are penicillin susceptible, although some are more susceptible than others. Most are exquisitely sensitive to penicillin G and have minimal inhibitory concentrations (MICs) of less than 0.12 mcg/mL (mg/L).4,19 Approximately 10% to 20% are moderately susceptible (MIC 0.12 to 0.5 mcg/mL [mg/L]). This difference in in vitro susceptibility led to recommendations that the MIC be determined for all viridans streptococci and that the results be used to guide therapy. Some streptococci are deemed tolerant to the killing effects of penicillin, where the minimal bactericidal concentration (MBC) exceeds the MIC by 32 times. A tolerant organism is inhibited but not killed by an antibiotic normally considered bactericidal.4 Bactericidal activity is required for successful treatment of infective endocarditis; therefore, infections with a tolerant organism may relapse after treatment. Despite some animal studies of endocarditis suggesting that tolerant strains do not respond as readily to β-lactam therapy as nontolerant ones, this phenomenon is primarily a laboratory finding with little clinical significance.4,19 Treatment for tolerant strains is identical to that for nontolerant organisms, and measurement of the MBC is not recommended.4
An assortment of regimens can be used to treat uncomplicated NVE caused by fully susceptible viridans streptococci (Table 89–4). Two single-drug regimens consist of high-dose parenteral penicillin G or ceftriaxone for 4 weeks. If a shorter course of therapy is desired, the guidelines suggest either high-dose parenteral penicillin G or ceftriaxone in combination with an aminoglycoside.4 When used in select patients, this combination is as effective as 4 weeks of penicillin alone. Although streptomycin was listed in previous guidelines, gentamicin is the preferred aminoglycoside because serum drug concentrations are obtained easily, clinicians are more familiar with its use, and the few strains of streptococci resistant to the effects of streptomycin–penicillin remain susceptible to gentamicin–penicillin. Other aminoglycosides are not recommended.
The decision of which regimen to use depends on the perceived risk versus benefit. For example, a 2-week course of gentamicin in an elderly patient with renal impairment may be associated with ototoxicity, worsening renal function, or both. Furthermore, the 2-week regimen is not recommended for patients with known extracardiac infection. On the other hand, a 4-week course of penicillin alone generally entails greater expense, especially if the patient remains in the hospital. Monotherapy with once-daily ceftriaxone offers ease of administration, facilitates home healthcare treatment, and may be cost-effective.1,4,25
The British Society for Antimicrobial Chemotherapy guidelines suggest that all of the following conditions be present to consider a 2-week treatment regimen for penicillin-sensitive streptococcal endocarditis35:
1. Penicillin-sensitive viridans streptococcus or S. bovis (penicillin MIC <0.1 mcg/mL [mg/L])
2. No cardiovascular risk factors such as heart failure, aortic insufficiency, or conduction abnormalities
3. No evidence of thromboembolic disease
4. Native valve infection
5. No vegetation of greater than 5 mm diameter on echocardiogram
6. Clinical response within 7 days (the temperature should return to normal, the patient should feel well, and the patient’s appetite should return to normal)
![]() When a patient has a history of an immediate-type hypersensitivity to penicillin, vancomycin should be chosen for infective endocarditis caused by viridans streptococci. When vancomycin is used, the addition of gentamicin is not recommended.4 Most patients who report a penicillin allergy have a negative penicillin skin test and consequently are at low risk of anaphylaxis.38 The published experience with penicillin is more extensive than with alternative regimens; consequently, a thorough allergy history must be obtained before a second-line therapy is administered.
When a patient has a history of an immediate-type hypersensitivity to penicillin, vancomycin should be chosen for infective endocarditis caused by viridans streptococci. When vancomycin is used, the addition of gentamicin is not recommended.4 Most patients who report a penicillin allergy have a negative penicillin skin test and consequently are at low risk of anaphylaxis.38 The published experience with penicillin is more extensive than with alternative regimens; consequently, a thorough allergy history must be obtained before a second-line therapy is administered.
For patients with complicated infections (e.g., extracardiac foci) or when the streptococcus has an MIC of 0.12 to less than or equal to 0.5 mcg/mL (mg/L), combination therapy with an aminoglycoside for the first 2 weeks and penicillin (higher dose) or ceftriaxone is recommended, followed by penicillin or ceftriaxone alone for an additional 2 weeks (Table 89–4).4 Some viridans streptococci have biologic characteristics that complicate diagnosis and treatment, previously referred to as nutritionally variant streptococci. Abiotrophia defectiva and Granulicatella species have nutritional deficiencies that hinder growth in routine culture media.4,5,19 These organisms require special broth supplemented with pyridoxal hydrochloride or cysteine. For patients infected with nutritionally variant streptococci or when the Streptococcus has an MIC of more than 0.5 mcg/mL (mg/L), treatment should follow the enterococcal endocarditis treatment guidelines.4
The rationale for combination therapy of penicillin-susceptible viridans streptococci is that enhanced activity against these organisms usually is observed when cell-wall–active agents are combined with aminoglycosides in vitro.39 Combined treatment results in quicker sterilization of vegetations in animal models of endocarditis and probably explains the high response rates observed for patients treated for a total of 2 weeks.4,40 The combined treatment, however, is not superior to penicillin alone. Some authors question the need for combination therapy in relatively resistant streptococci, emphasizing that few human data suggest that patients with endocarditis caused by these organisms respond less well to penicillin alone.39,41
For patients with endocarditis of prosthetic valves or other prosthetic material caused by viridans streptococci and S. bovis, choices of treatment are similar to those without prosthetic material (e.g., penicillin or ceftriaxone); however, treatment courses are extended to 6 weeks (Table 89–5). In fact, if the organism is relatively resistant, gentamicin is recommended for 6 weeks.
Whether extended-interval aminoglycoside dosing has a role in infective endocarditis continues to be debated. At this time, data support extended-interval dosing for the treatment of streptococcal infective endocarditis, and as compared with three-times-daily dosing this approach may have greater efficacy.42–45 One study specifically evaluated the combination of ceftriaxone (2 g daily) with gentamicin (3 mg/kg daily) for 2 weeks compared with ceftriaxone (2 g daily) alone for 4 weeks for penicillin-sensitive streptococci. Both regimens were safe and effective with similar clinical cure rates at 3 months following treatment.40



