Chapter 18 Infectious diseases of the skin
Viral infections
Common wart
The common wart (verruca vulgaris) is caused by infection with human papillomavirus (HPV) (Fig. 18.1). HPV is a DNA virus of the papovavirus family. The number of known HPV genotypes currently stands at more than 100, classified according to the extent of their DNA homology (DNA hybridization) (Table 18.1).1–4 In order for an HPV type to be regarded as ‘new’, sequences in selected genomic regions must exhibit more than 10% divergence compared to any of the known HPV types.4 Monoclonal antibodies to intact viruses have been produced and can demonstrate individual types of HPV; antibodies to viral components are only group specific (Fig. 18.2). In recent years, advances in molecular pathology have resulted in improved and more specific methods of HPV detection, including in situ polymerase chain reaction (PCR) and nonisotopic in situ hybridization (NISH).5
Table 18.1 Variants of human wart virus infection
| HPV type | Associated clinical lesions |
|---|---|
| 1 | Deep plantar warts, common warts |
| 2 | Common warts, flat warts |
| 3 | Flat warts |
| 4 | Common warts, plantar warts |
| 5 | Epidermodysplasia verruciformis (EV) |
| 6 | Genital warts, laryngeal papilloma |
| 7 | Butcher warts |
| 8 | EV |
| 9 | EV, keratoacanthoma |
| 10 | Flat warts |
| 11 | Laryngeal papillomas, genital warts |
| 12 | EV |
| 13 | Focal epithelial hyperplasia |
| 14, 15 | EV |
| 16 | Genital warts, bowenoid papulosis, cervical dysplasia, cervical carcinoma, digital, squamous cell carcinoma, nongenital Bowen’s disease |
| 17 | EV |
| 18 | Genital warts, bowenoid papulosis, cervical dysplasia, cervical carcinoma |
| 19–25 | EV, keratoacanthoma |
| 26–29 | Common warts, flat warts |
| 30 | Laryngeal carcinoma, genital warts |
| 31–32 | Genital warts, bowenoid papulosis, cervical dysplasia, cervical carcinoma |
| 33 | Cervical carcinoma |
| 34 | Bowenoid papulosis, Bowen’s disease |
| 35 | Cervical dysplasia, cervical carcinoma |
| 36 | EV |
| 37 | EV, keratoacanthoma |
| 38 | EV |
| 39 | Bowenoid papulosis, cervical carcinoma |
| 41 | Flat warts |
| 42 | Genital warts, bowenoid papulosis, cervical dysplasia, cervical carcinoma |
| 43, 44 | Genital warts, laryngeal papillomas |
| 46, 47 | EV |
| 48 | Bowenoid papulosis, Bowen’s disease |
| 49, 50 | EV |
| 51–54 | Genital warts, bowenoid papulosis, cervical dysplasia, cervical carcinoma |
| 55 | Genital warts, laryngeal papillomas |
Reproduced with permission from Melton, J.L. and Rasmussen, J.E. (1991) Dermatologic Clinics, 9, 219–233.
Papillomaviruses, which are small, nonenveloped, and show icosahedral symmetry, contain circular double-stranded DNA composed of approximately 8000 base pairs.4,6 The viral particle, which has a diameter of approximately 55 nm, contains 72 capsomeres.1,2,4,6 The HPV genome is divided into three functional regions: a late region, an early region, and a noncoding 1000 base pair upstream regulatory region (URR). The URR is located immediately upstream of the E6 open reading frame (ORF) and contains sequences regulating expression of all ORFs, including promoter elements and transcriptional enhancer sequences. In excess of 20 messenger RNAs are expressed, usually in a differentiation-specific and cell-specific manner.4 Genes in the early region (E1, E2, E4, E5, E6, E7) are responsible for transcription, replication, and cellular transformation. The E4 ORF is highly expressed in differentiated HPV-infected epithelial cells. Some forms of E4 encode a protein capable of disrupting the cytokeratin network, resulting in the phenomenon of koilocytosis.4 The E4 ORF represents a region of maximal divergence between different HPV types.7 Each viral genotype is most often detected in lesions at specific anatomical sites or shows distinct histological characteristics.2,8–10
HPV infection in man results in a variety of lesions including verruca vulgaris, filiform warts, verruca plana, plantar warts, anogenital warts, and bowenoid papulosis.6 Mucosal lesions include oral warts and condylomata, focal epithelial hyperplasia or Heck’s disease, nasal and conjunctival papillomas, laryngeal papillomatosis, and cervical lesions.4,6 In addition to its role in cervical cancer, HPV is increasingly recognized as an important cause of a number of neoplasms including Bowen’s disease and malignant lesions of the anus, external genitalia, and elsewhere.2 Of equal importance is the recognition that HPV infection may be asymptomatic or result in a carrier status. A recent study showed that cutaneous HPV infections commonly persist on healthy skin over several years, and that persistence does not appear to be associated with age, sex, a history of warts, immunosuppressive therapy, or HPV type.11
Clinical features
Common warts are caused by HPV types 1, 2, 4, 7, and 26–29.2,6 In immunosuppressed patients, HPV subtypes 75, 76, and 77 may be pathogenetic.12 A case with extensive, recalcitrant verrucae linked to infection with HPV type 57 has been reported.13 Rarely, HPV subtypes associated with genital warts such as 6 and 11 have been found in common warts in children.14 HPV-16, a common genital HPV type with oncogenic potential, was detected in 6.6% of lesions in a recent series of 45 immunocompetent patients with nongenital cutaneous warts.15 Conversely, verrucae vulgaris may sometimes occur on the vulva. HPV type 2 has been detected in such cases. It is important to note that these ‘nonvenereal’ genital lesions may occur in girls less than 5 years of age, as an erroneous clinical or histological diagnosis of condylomata acuminata could lead to allegations of sexual abuse.16
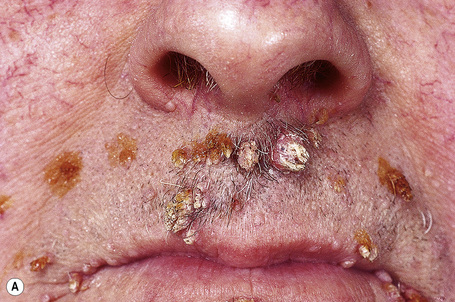
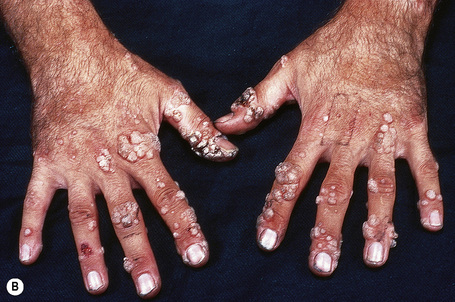
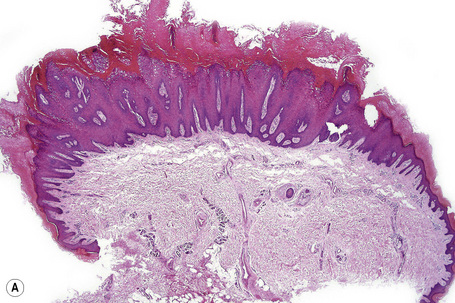
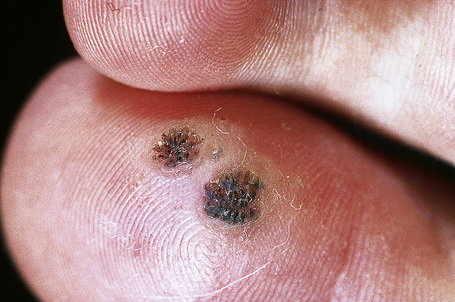
Warts are very common lesions, particularly in children.17 Adults are also frequently affected. In a survey of 2180 adults, 3.5% had warts.18 Butchers and slaughterhouse workers have an increased risk.19,20 Common warts may occur anywhere on the skin and in people of any age, but are most common on the backs of the hands and the fingers and on the knees of young children, where they appear as firm keratotic papules 1–10 mm across (Figs 18.3, 18.4).17 Koebnerization is common (hence kissing lesions on fingers).6In other sites they may appear more filiform and less firm (Fig. 18.5). The latter are particularly seen on the lips, nostrils, and eyelids.3 Giant periungual lesions have been described.21 Warts may also present as a cutaneous horn. They persist for a few months up to several years and often regress spontaneously, particularly in children.
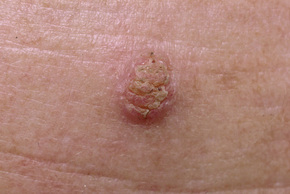
Fig. 18.3 Verruca vulgaris: there is a raised, discrete scaly plaque on the dorsal aspect of the hand.
From the collection of the late N.P. Smith, MD, the Institute of Dermatology, London, UK.
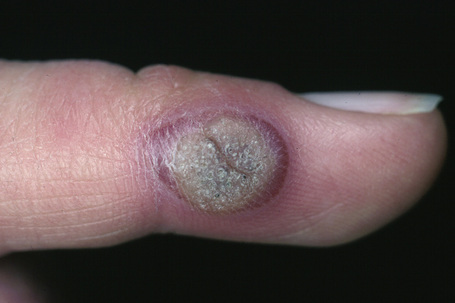
Fig. 18.4 Verruca vulgaris: verrucae are most commonly seen on the hands and fingers.
From the collection of the late N.P. Smith, MD, the Institute of Dermatology, London, UK.
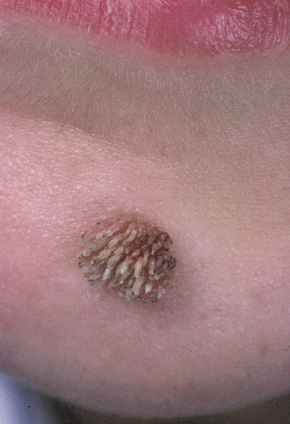
Fig. 18.5 Filiform wart: this variant occurs most often on the face and around the axillae.
From the collection of the late N.P. Smith, MD, the Institute of Dermatology, London, UK.
Chronically immunosuppressed patients (e.g,. following renal transplantation) often have a large number of warts (Fig. 18.6).22,23 Chronicity is associated with increasing numbers of lesions. Epidermodysplasia verruciformis-like lesions due to HPV-5 have also been described in HIV-positive patients and following renal transplantation.24 Numerous warts may be seen in other immunosuppressed patients (e.g., with lymphoma, leukemia, Hodgkin’s lymphoma, and HIV infection).6,25–27 In patients with AIDS, warts may regress following highly active antiretroviral therapy (HAART).28
Pathogenesis and histological features
In situ hybridization studies of HPV lesions have shown that viral DNA synthesis in the epidermis occurs in the superficial prickle cell layer and full virus assembly with capsid production occurs in the granular cell layer.6 HPV DNA has been demonstrated in apparently normal skin up to 15 mm from a virus-associated lesion.29 The requirement for growth in very well-differentiated epithelia may explain the difficulty of culturing HPV and why host destruction of the lesions may be protracted. Immune mechanisms are presumed to be less effective against organisms or altered cells that are situated superficially with no direct blood supply.
Regression of HPV lesions is usually spontaneous, but may not occur for several years.30 Cell-mediated immunity seems to be important in effecting the regression since lymphocytes are seen infiltrating the wart epithelium at this stage. Other features of regression include liquefactive basal cell degeneration, epidermal degeneration, and vascular thrombosis.30,31 Recently, Toll-like receptors (TLRs) have been identified as important role players in viral recognition and the initiation of an antiviral host immune response. TLR3, TLR9, IFN-β and TNF-α appear to play an important role in the skin’s innate immune response to HPV infection.32 Langerhans cells and Langerhans-like dendritic cells may exert direct antiviral activity. This is facilitated via the expression of TLRs such as TLR3, which may in turn trigger the release of IFN-inducible chemokines, including CXCL9, a monokine induced by IFN-γ.33
Transmission of HPV is by inoculation of infected desquamated cells through close contact at points of minor trauma; hence common warts are seen most often on the hands. Periungual warts are particularly associated with nail biting and plantar warts are especially related to prolonged immersion in water.18
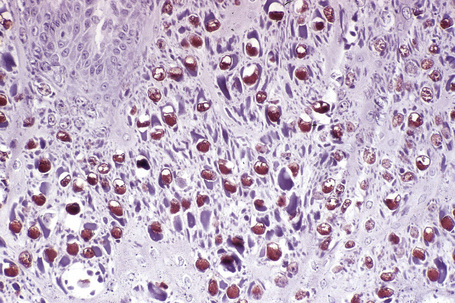
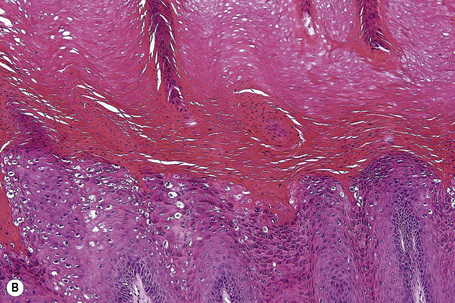
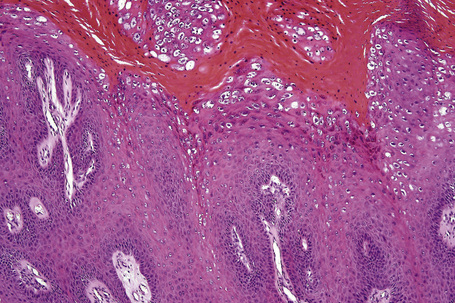
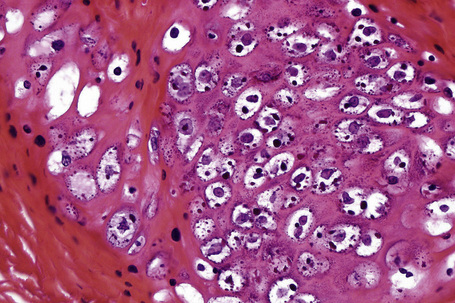
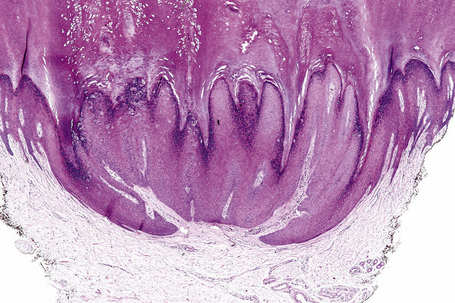
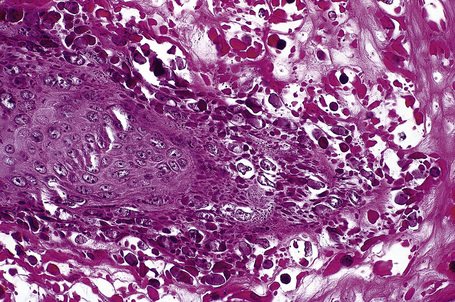
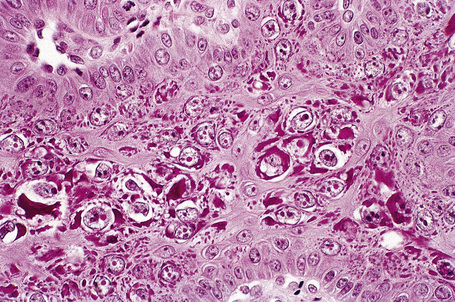
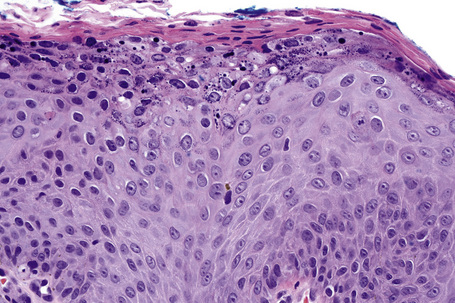
Common warts show filiform acanthosis with vertical tiers of parakeratosis over the tips of the exophytic component (Fig. 18.7). There is also marked orthokeratosis. A downward extension of the acanthosis produces a curvilinear deep margin and curved distortion of the adjacent rete ridges in the uninvolved epidermis. There is a prominent granular cell layer within which are enlarged clumps of irregular basophilic keratohyalin (Fig. 18.8).3 These are seen best in the concavities between the papillomatotic epithelial papillae. Large cells with prominent vacuolated cytoplasm and a small pyknotic nucleus are seen in the upper layers of the epidermis (koilocytes) (Fig. 18.9). Koilocytes are, however, more frequently observed in genital warts (see below). Connective tissue and tortuous small blood vessels may invade the filiform projections (Fig. 18.10). In some cases, involvement of the superficial portion of the hair follicles by HPV results in focal changes identical to a trichilemmoma or an inverted follicular keratosis.34 However, not all of these lesions are induced by HPV as has been suggested.35
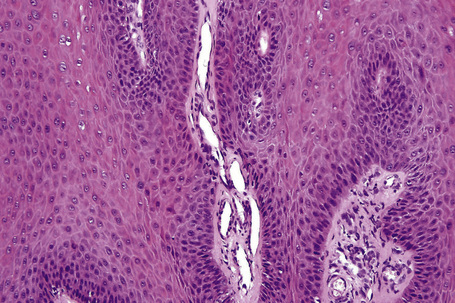
Fig. 18.10 Verruca vulgaris: the core of the papillary projection contains conspicuous dilated capillary loops.
Ordinary common warts are only exceptionally associated with in situ or invasive squamous cell carcinoma.36,37 HPV-16 has been associated with periungual Bowen’s disease and squamous carcinoma.38–40 The role of HPV in cutaneous neoplasia is discussed further in Chapter 22. Recent molecular studies have implicated cutaneous HPV infection as a carcinogenic cofactor in association with solar ultraviolet radiation in the evolution of nonmelanoma skin cancer.41
Plantar warts
Clinical features
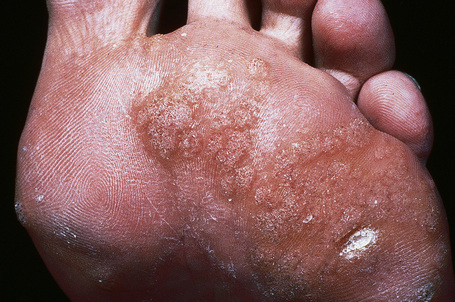
Plantar warts occur on the sole of the foot; they are only slightly elevated and appear as a horny plug surrounded by a ring of hyperkeratotic skin (Fig. 18.11). Often they are covered with black dots representing thrombosed capillaries (Fig. 18.12).1 They are most common in children and are frequently seen over pressure points. Most plantar warts are caused by HPV-1 and are painful; however, HPV-4 may produce a confluent or mosaic pattern of similar small warts (‘mosaic plantar warts’) and these are painless (Fig. 18.13).2,3 They may also be seen on the palms and in the periungual region. There have been reports from Japan of unusual plantar warts produced by HPV-60.4–6
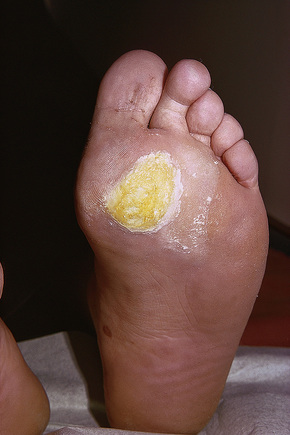
Fig. 18.11 Plantar wart: the lesion is flat and shows very marked hyperkeratosis.
By courtesy of R.A. Marsden, MD, St George’s Hospital, London, UK.
The lesions may be nodular, ridged or pigmented. A cystic variant has also been described.4–10 The cystic variant has the features of an epidermoid cyst and may rarely be multiple.11 Most are associated with HPV-60 but an association with HPV-57 has also been reported.12–14 Epidermoid cysts induced by HPV may also be seen outside acral locations.15,16 Pigmented warts are caused by HPV-4, 60 or 6517 and may contain fibrillar intracytoplasmic inclusion bodies.18 A case of a large plantar wart caused by HPV-66 has been documented.19 A further subtype of HPV associated with palmoplantar warts is HPV-63.8,20
Plantar warts usually regress within a few months in children, but may persist longer in adults. Rarely, chronic plantar warts may be associated with the development of verrucous carcinoma (carcinoma cuniculatum) (see Ch. 22).21,22
Histological features
Plantar warts are almost entirely endophytic, with a central parakeratotic plug surrounded by multiple deep extensions of acanthotic epidermis (Fig. 18.14). The depth and complexity of these downgrowths have been likened to an anthill, giving rise to the term ‘myrmecia’. Vacuolation is more prominent in the plantar wart and, in the active growing phase, large eosinophilic (and to a lesser extent basophilic) cytoplasmic inclusions are present, which represent disordered growth of giant keratohyalin granules (Fig. 18.15). The large eosinophilic cytoplasmic inclusions are usually seen in infections caused by HPV-1 and to a lesser extent in those caused by HPV-60 and 65.7 In warts induced by HPV-4, the infected keratinocytes show prominent cytoplasmic vacuolar change with almost no keratohyalin granules. Intranuclear inclusions may also be evident (Fig. 18.16). HPV can be demonstrated in the nuclei of these cells by electron microscopy (Fig. 18.17). Melanin granules are discernible within the cytoplasm of HPV-60-induced pigmented plantar warts.
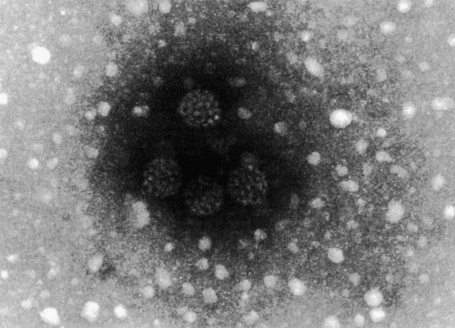
Fig. 18.17 Plantar wart: this honeycomb arrangement of HPV is characteristic.
By courtesy of I. Chrystie, FIMLS, St Thomas’ Hospital, London, UK
Regressive changes are the same as those described in common warts and consist of thrombosis of superficial blood vessels, necrosis, and a mixed inflammatory cell infiltrate.23 A recently described multiplexed PCR-based assay may have merit in both HPV genotyping and in monitoring treatment efficacy.24
Plane warts
Clinical features
Plane warts (verrucae plana), usually caused by HPV-2, 3 or 10, are flat, smooth, and a few millimeters in diameter with typically little change in color from the adjacent skin, although they may appear gray-yellow or pale brown (Fig. 18.18).1–3 HPV-5 is rarely implicated in HIV-infected patients.4 Plane warts may also result from HPV types 26–29 and 41 infection.5 They affect the face, backs of the hands, and the shins. There may be only a few present, but occasionally they are very numerous and become confluent in areas of scratching (koebnerization).6 Plane warts are common in children and may be seen in women, but are not usually found in males after puberty except in association with HIV infection. They may regress spontaneously after a few weeks or months, or may persist for years. Signs of regression include pruritus, an erythematous, edematous appearance, depigmented haloes, and an eruption of multiple tiny plane warts.2,6,7 Cell-mediated immunity plays a key role in the spontaneous regression of plane warts in immunocompetent individuals.8 Multiple plane warts may evolve as a cutaneous manifestation of immune reconstitution inflammatory syndrome (IRIS) in HIV-infected patients receiving highly active antiretroviral therapy.9 Exacerbation of lesions has been reported following facial laser resurfacing.10
Histological features
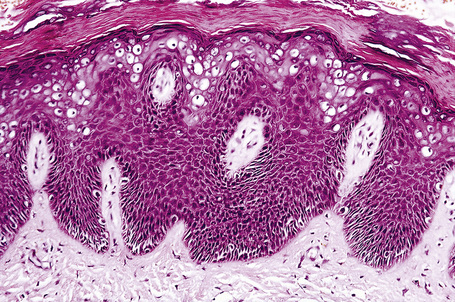
Plane warts are acanthotic and show orthokeratosis with an open pattern reminiscent of ‘chicken wire’ (‘basket weave’ hyperkeratosis). Parakeratosis is not a feature and there is little papillary configuration to the acanthosis (Fig. 18.19). Keratinocytes of the upper part of the stratum spinosum show striking cytoplasmic vacuolation with margination of the keratohyalin granules and tonofilaments.4 Regression is characterized by keratinocyte necrosis (apoptosis), individual cell keratinization, parakeratosis, lymphocytic exocytosis with spongiosis, and a superficial perivascular chronic inflammatory cell infiltrate.2,11–13 The lymphocytes encountered in regressing lesions have been found to express the cytotoxic granule granzyme-B.14 Extravasation of erythrocytes may be a feature and edema of the papillary dermis is frequently present.15
Condyloma acuminatum
Clinical features
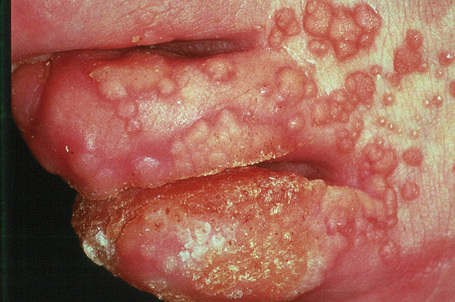
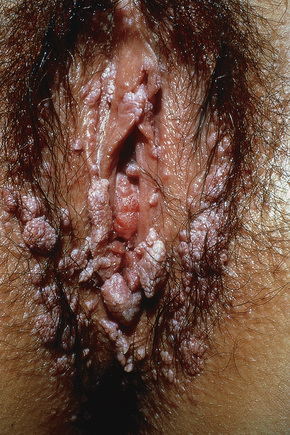
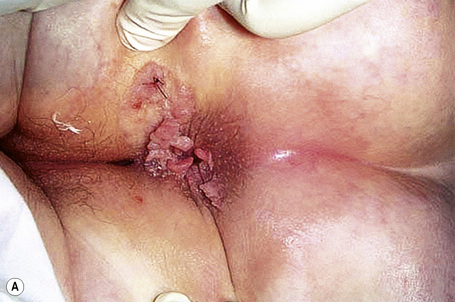
Condylomata acuminata are particularly caused by HPV types 2, 6, 11, 16, 18, 30–33, 35, 39, 41–45, 51–56, and 59 and develop as a consequence of the trauma accompanying sexual intercourse.1–13 HPV-6 and 11 alone account for more than 90% of these lesions, with HPV-6 present in about two-thirds of cases and the remaining one-third caused by HPV-11.4,5 The incubation period is variable (usually between 2 and 3 months).14 Condylomata acuminata occur on the glans penis and prepuce or shaft as soft, fleshy, sometimes filiform plaques and may extend into the meatus (Figs 18.20, 18.21). On the shaft they are less exophytic. Vulval lesions may be bulky and macerated, and may extend into the introitus (Fig. 18.22). Similar fleshy and filiform soft masses occur perianally, more often in males (Figs 18.23, 18.24). Anal squamous carcinoma has also been shown to contain HPV-6, 16, and 18 in a significant proportion of cases (Fig. 18.25).6 The rate of local recurrence is about 30%.15 The lesions are uncommon in children (when they may be a sign of sexual abuse) and are seen most often in young adults (second and third decades), frequently in association with other genital infections.4,9,16 Childhood condylomata regress spontaneously in more than 50% of cases.17
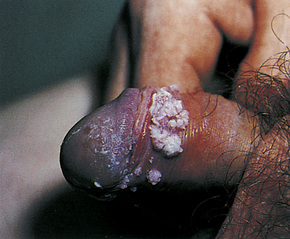
Fig. 18.20 Condyloma acuminatum: note the typical filiform appearance.
By courtesy of the Department of Genitourinary Medicine, St Thomas’ Hospital, London, UK.
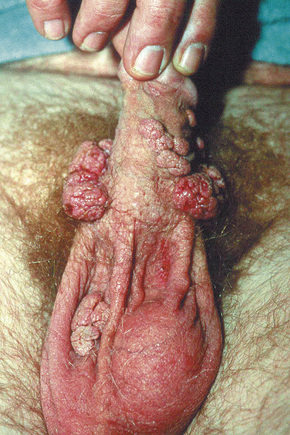
Fig. 18.21 Condyloma acuminatum: there are multiple lesions on the shaft of the penis and scrotum.
By courtesy of the Department of Genitourinary Medicine, St Thomas’ Hospital, London, UK.
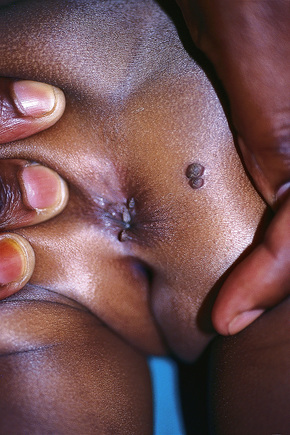
Fig. 18.23 Condyloma acuminatum: multiple perianal lesions are present.
By courtesy of N.C. Dlova, MD, Nelson R. Mandela School of Medicine, University of KwaZulu-Natal, South Africa.
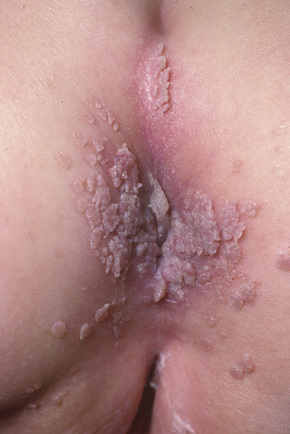
Fig. 18.24 Condyloma acuminatum: there is very extensive involvement of the perineum.
From the collection of the late N.P. Smith, MD, the Institute of Dermatology, London, UK.
It is important to note that a significant proportion of genital HPV infections are asymptomatic.3,18 The female partners of male patients with condyloma acuminata have been shown to have an increased risk of cervical HPV infection and intraepithelial neoplasia (SIL/CIN).19 Cervical neoplasia associated with pre-existent condylomata acuminata has also been related to a background of immunosuppression, at least in some patients.20 The worldwide HPV prevalence in cervical carcinomas is reported to be 99.7%.21 HPV-16, 18, 31–33, 35, 39, 42, and 51–54 are most commonly associated with cancers of the cervix, vulva, and penis.7–9,22,23 According to a recent study, patients with condylomata acuminata are at increased risk for developing not only carcinomas of the vulva, vagina, penis, and anus, but also certain nonanogenital squamous cell carcinomas.24 It is anticipated that routine vaccination with a quadrivalent vaccine against HPV types 6, 11, 16, and 18 will lead to a significant reduction in the burden of vulval and cervical carcinomas, genital warts, and genital intraepithelial neoplasia.25
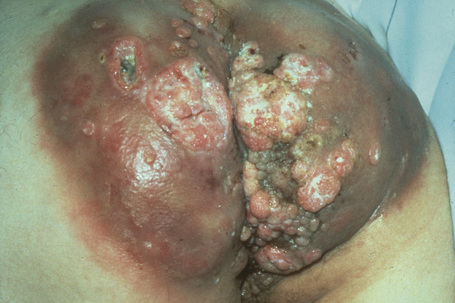
A large, exuberant, and locally destructive variant of condyloma (Buschke-Löwenstein tumor) may rarely be encountered (Fig. 18.26).26,27 This is associated with HPV types 6, 11 or 16. It is likely that this giant variant represents a variant of verrucous carcinoma but the issue has been controversial (see Ch. 22).26–30 Juvenile laryngeal papillomas containing HPV-6 and 11 can be seen in children born to mothers with condylomata acuminata.3 They may show malignant progression if irradiated.
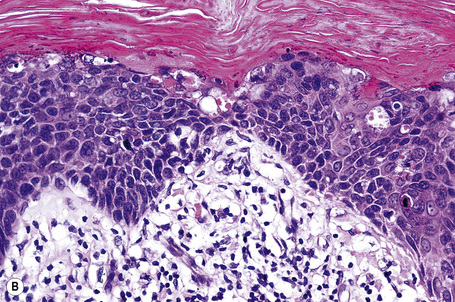
Histological features
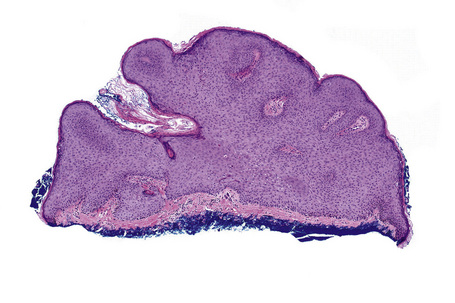
Condylomata acuminata are characterized by marked acanthosis with a solid or trabecular pattern and a broad rounded exophytic growth (Fig. 18.27). There is a sharp, fairly regular, deep margin. The surface of the lesion is hyperkeratotic and parakeratotic. Superficial vacuolated keratinocytes (koilocytes) are characteristic (Fig. 18.28) and coarse keratohyaline granules may be present. The vacuolated epithelium is often most marked in the declivities. Condylomata that are treated with podophyllin prior to removal demonstrate marked epidermal pallor and increased mitoses and necrotic keratinocytes in the lower half of the epidermis.31 These changes may lead to a misdiagnosis of malignancy. Giant condyloma acuminatum (anogenital verrucous carcinoma, Buschke-Löwenstein tumor) occurs most frequently on the genitalia, and is larger and more cauliflower-like.26–28 It shows some tendency to endophytic growth, but without any suggestion of frank infiltration. It can recur locally, but metastasizes very rarely. Most experts regard this lesion as a variant of verrucous carcinoma. Anal condylomata may develop bowenoid features, and occasionally invasive tumor supervenes.6–8
Bowenoid papulosis
Clinical features
Bowenoid papulosis (koilocytosis with intraepithelial neoplasia) is a clinicopathological entity that bears marked histological similarity to koilocytosis, SIL/CIN, and Bowen’s disease. Although the term is no longer used by The International Society for Study of Vulval Disease (ISSVD), many clinicians believe that it represents a distinctive clinicopathological entity and we have decided to describe it in this chapter. Clinically, it is quite different from genital Bowen’s disease in that multiple small papules develop over a short time scale in young people. Prognosis is uncertain; many patients do not show evidence of progression, but a small proportion may develop invasive tumor and, on occasion, this may have metastatic potential. It is usually associated with HPV-16 or 18, but occasionally HPV types 31–35, 39, 42, 49, and 51–54 are detected.1–6 Although uncommon, some cases may be associated with mixed infection by different HPV types.6,7 A unique HIV-associated case with genital and extragenital (lip) lesions caused by two separate HPV types (HPV-16 and HPV-32, respectively) has been described.8 E6 and E7 viral oncoproteins of high-risk HPV types induce overexpression of p16 and human telomerase reverse transcriptase.9
Bowenoid papulosis most often presents as multiple reddish-brown, sometimes lichenoid, discrete papules, but occasionally these become a confluent plaque. Papules, on average 4 mm in diameter, are found on the penis, vulva, perianal region, and perineum. Extragenital sites of occurrence include the face, neck, and fingers.10–12 An isolated case of oral bowenoid papulosis in an HIV-infected male has been reported.13 Bowenoid papulosis manifests in young, sexually active adults in contrast to true Bowen’s disease, which occurs in an older age group. Genital Bowen’s disease is, however, also often associated with HPV-16.14 The occurrence in childhood should raise suspicion of sexual abuse.3 Genital bowenoid papulosis has been associated with periungual bowenoid dysplasia.15
Spontaneous regression is uncommon.16 As progression to frank invasive carcinoma in bowenoid papulosis is rare, these lesions are best managed conservatively. However, bowenoid papulosis may be resistant to treatment and may be characterized by a prolonged course in immunosuppressed patients.2 Bowenoid papulosis has also been associated with oral warts and lingual carcinoma.4 It has recently been shown that patients with bowenoid papulosis and HPV infection may be primarily immunosuppressed due to diminished T-helper cell levels (non-HIV-associated).2,14 The condition may also occur in organ transplant recipients.17 Penile bowenoid papulosis is associated with a high risk of the consort developing cervical dysplasia.18,19 Consequently, female patients and consorts should regularly have cervical smears.
Histological features
A bowenoid papulosis lesion consists of a well-circumscribed area of acanthosis producing a raised plaque or dome, which is hyperkeratotic and sometimes shows superficial epithelial vacuolation.20,21 The keratinocytes may show nuclear hyperchromatism and pleomorphism. There is variable dyskeratosis.
Epidermodysplasia verruciformis
Clinical features
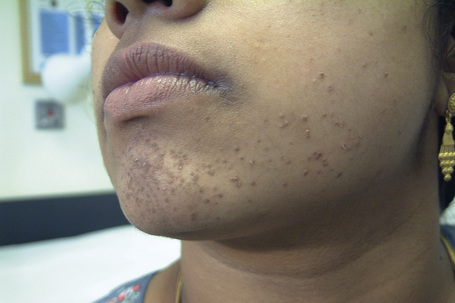
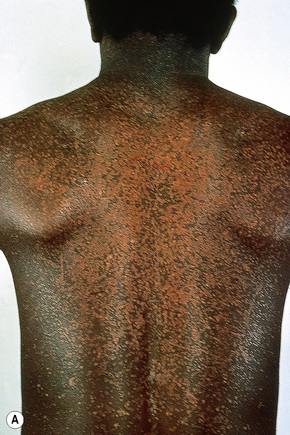
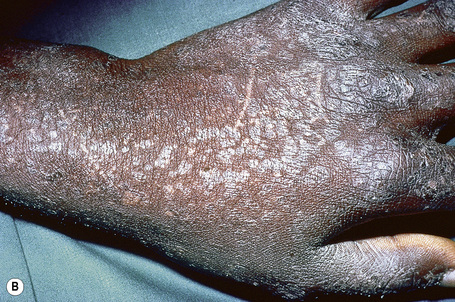
Epidermodysplasia verruciformis (EV) is a rare inherited condition characterized by selective susceptibility to skin infection with certain HPV types, defects in cell-mediated immunity, and an increased risk for the development of cutaneous malignancies, especially squamous cell carcinomas.1,2 Affected individuals present with a wide range of HPV subtypes including 3, 5, 8–10, 12, 14, 15, 17, 19–25, 28, 29, 36–38, 46, 47, 49, 50, 51, and 59.1,3–5 The more common flat warts, caused by HPV-3 and HPV-10, may also occur in these patients but have an extensive distribution pattern; they may form plaques and can be persistent.6 These are seen most often on the arms, legs, face, and the dorsum of the hands (Figs 18.29–18.31).4 The specific EV subtypes of HPV cause reddish, or pigmented or depigmented, scaly flat macular plane warts, mainly on the trunk, but also on the face, neck, and arms.2 Clinically they resemble pityriasis versicolor (Fig. 18.32). Some patients, especially those who are dark-skinned, may present with seborrheic keratosis-like changes.7,8 Spiny hyperkeratosis of the fingers is a rare manifestation.9
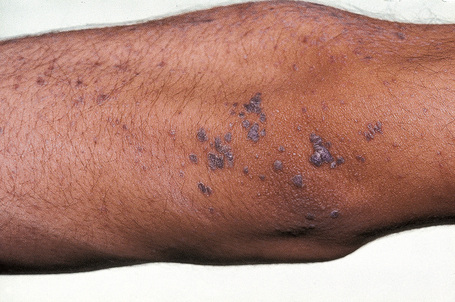
Fig. 18.30 Epidermodysplasia verruciformis: these plane warts are due to HPV-3 and HPV-10 infection.
By courtesy of M.M. Black, MD, Institute of Dermatology, London, UK.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree