Some bacteria develop a resistance to antibiotics.
Some microorganisms, such as human immunodeficiency virus, include many different strains and a single vaccine can’t provide protection against them all.
Most viruses resist antiviral drugs.
Some microorganisms localize in areas that make treatment difficult, such as the central nervous system and bone.
sometimes for years. An exogenous infection results from environmental pathogens or sources other than the host; an endogenous infection results from the host’s normal flora (for instance, Escherichia coli displaced from the colon, which causes urinary tract infection).
 |
 |
 |
or nonmotile bacteria); their tendency toward encapsulation (encapsulated or nonencapsulated bacteria); and their capacity to form spores (sporulating or nonsporulating bacteria).
In contact transmission, the susceptible host comes into direct contact (as in contact with blood or body fluids) or indirect contact (contaminated inanimate objects or the close-range spread of respiratory droplets) with the source. The most common method of contact transmission is contaminated hands.
Airborne transmission results from the inhalation of contaminated aerosolized droplet nuclei (as in pulmonary tuberculosis).
In enteric (oral-fecal) transmission, the infecting organisms are found in feces and are ingested, in many cases through fecally contaminated food or water (as in salmonella infections).
Vector-borne transmission occurs when an intermediate carrier (vector), such as a flea, mosquito, or other animal, transfers an organism.
comprehensive immunization (including required immunization of travelers to, or emigrants from, endemic areas)
drug prophylaxis
improved nutrition, living conditions, and sanitation
correction of environmental factors
widespread disease tracking
passive immunity. Generally, passive immunization is used when active immunization is perilous or impossible or when complete protection requires both active and passive immunization. It may also be appropriate in situations requiring immediate protection such as postexposure in which active immunity from immunizations takes too long to provide the necessary and immediate protection. Maternal passive immunity crosses the placental barrier from mother to fetus and is also provided to the infant by antibodies present in breast milk.
Wear gloves when touching blood and body fluids, mucous membranes, or the broken skin of patients; when handling items or touching surfaces soiled with blood or body fluids; and when performing venipuncture and other vascular access procedures.
Change gloves and wash hands after contact with each patient.
Wear a mask and protective eyewear, or a face shield, to protect the mucous membranes of the mouth, nose, and eyes during procedures that may generate the splatter of blood or other body fluids.
In addition to the mask and protective eyewear or face shield, wear a gown or an apron during procedures that are likely to cause splashing of blood or other body fluids.
After removing gloves and other protective equipment, thoroughly wash hands and other skin surfaces that may be contaminated with blood or other body fluids.
During all invasive procedures, wear gloves and a surgical mask and goggles or a face shield as appropriate.
During procedures that commonly cause droplets or splashes of blood or other body fluids, or for those that generate bone chips, wear protective eyewear and a surgical mask or a face shield.
During invasive procedures that are likely to cause splashing or a splattering of blood or other body fluids, wear a gown or an impervious apron.
If performing or assisting in a vaginal or cesarean delivery, wear gloves and a gown when handling the placenta or the infant and during umbilical cord care.
During spinal procedures (lumbar puncture, spinal and epidural anesthesia, myelogram), wear a face mask to prevent droplet spread of oral flora.
To prevent needle-stick injuries, don’t recap used needles, bend or break needles, remove needles from their disposable syringes or phlebotomy blood tube holders, or manipulate them.
Whenever possible use single-dose vials over multi-dose vials, especially when medications will be administered to multiple patients.
Use a sterile, single-use, disposable syringe and needle for each injection.
Use sharps safety devices. Activate safety mechanisms as directed.
Place disposable syringes and needles, scalpel blades, and other sharps items in punctureresistant containers for disposal. Make sure these containers are always located near the area of use.
Place large-bore reusable needles in a puncture-resistant container for transport to the reprocessing area immediately after a procedure.
If a glove tears or a needle-stick or other injury occurs, remove the gloves, wash your hands and the site of the needle-stick thoroughly, and put on new gloves as quickly as patient safety permits. Remove the needle or instrument involved in the incident from the sterile field. Promptly report injuries and mucous-membrane exposure to the appropriate infection control practitioner per facility protocol.
when hands are visibly dirty or contaminated with proteinaceous material or visibly soiled with blood or other body fluids (even if gloves were worn)
before eating and after using the restroom
exposure to suspected or proven Bacillus anthracis (alcohol, chlorhexidine, iodophors, and other antiseptic agents have a poor potency against its spores)
after caring for a patient with Clostridium difficile (alcohol, chlorhexidine, iodophors, and other antiseptic agents are largely ineffective against its spores)
Make sure mouthpieces, one-way valve masks, resuscitation bags, and other ventilation devices are available in areas where the need for resuscitation is likely. Note: Saliva has not been implicated in human immunodeficiency virus transmission.
If you have any exudative lesions or weeping dermatitis, refrain from direct patient care and from handling patient care equipment until the condition resolves.
Respiratory hygiene/cough etiquette includes covering the mouth and nose with a tissue when coughing and prompt disposal of used tissues, using surgical masks on the coughing person when tolerated, and hand hygiene before and after contact with respiratory secretions.
Follow strict infection-control procedures. (See Standard precautions. See also CDC isolation precautions, page 802.)
blood
all body fluids, secretions, and excretions— except sweat—regardless of whether or not they contain visible blood
skin that is not intact
mucous membranes
Age | Immunization |
Birth | HepB |
1 to 4 months | HepB |
2 months | DTaP, HIB, IPV, PCV, Rota |
4 months | DTaP, HIB, IPV, PCV, Rota |
6 months | DTaP, HIB, PCV, Rota |
6 to 18 months | HepB, IPV |
12 to 15 months | HIB, MMR, PCV, HepA, varicella |
6 months to 18 years | Influenza (yearly) |
12 to 23 months | HepA |
15 to 18 months | DTaP, HepA |
4 to 6 years | DTaP, IPV, MMR, varicella |
11 to 12 years | HPV, Tdap, MCV4 |
15 years | Tdap, MCV4 |
Document hospital infections as they occur.
Identify outbreaks early, and take steps to prevent their spread.
Eliminate unnecessary procedures that contribute to infection.
Strictly follow necessary isolation techniques.
Observe all patients for signs of infection, especially those patients at high risk.
Always follow proper hand-hygiene technique and encourage other staff members to follow these guidelines as well.
Keep staff members and visitors with obvious infection and well-known carriers away from susceptible, high-risk patients.
Take special precautions with vulnerable patients, such as those with indwelling urinary catheters, mechanical ventilators, or I.V. lines and those recovering from surgery.
to infection. Notice if the patient is listless or uneasy, lacks concentration, or has any obvious abnormality of mood or affect.
aminoglycosides, erythromycin, tetracycline, and clindamycin.
People in contact with the patient should perform hand hygiene before and after patient care.
Good hand hygiene is the most effective way to prevent MRSA infection from spreading.
Use an antiseptic soap such as chlorhexidine. Bacteria have been cultured from worker’s hands washed with milder soap. One study showed that, without proper hand hygiene, MRSA could survive on health care workers’ hands for up to 3 hours. Chlorhexidine has a residual antimicrobial effect on the skin.
Predisposing factors | Signs and symptoms | Diagnosis | Treatment | Special considerations |
Bacteremia | ||||
▪ Infected surgical wounds ▪ Abscesses ▪ Infected I.V. or intraarterial catheter sites or catheter tips ▪ Infected vascular grafts or prostheses ▪ Infected pressure ulcers ▪ Osteomyelitis ▪ Parenteral drug abuse ▪ Source unknown (primary bacteremia) ▪ Cellulitis ▪ Burns ▪ Immunosuppression ▪ Debilitating diseases, such as chronic renal insufficiency or diabetes ▪ Infective endocarditis (coagulase-positive staphylococci) and subacute bacterial endocarditis (coagulase-negative staphylococci) ▪ Cancer (leukemia) or neutrophil nadir after chemotherapy or radiation | ▪ Fever (high fever with no obvious source in children younger than age 1), shaking chills, tachycardia ▪ Cyanosis or pallor ▪ Confusion, agitation, stupor ▪ Skin microabscesses ▪ Joint pain ▪ Complications: sepsis; shock; acute bacterial endocarditis (in prolonged infection; indicated by new or changing systolic murmur); retinal hemorrhages; splinter hemorrhages under nails and small, tender red nodes on pads of fingers and toes (Osler’s nodes); abscess formation in skin, bones, lungs, brain, and kidneys; pulmonary emboli if tricuspid valve is infected ▪ Prognosis depends on early diagnosis and treatment, and presence of other medical conditions | ▪ Blood cultures (two to four samples from different sites at different times): growing staphylococci and leukocytosis (usually 12,000 white blood cells [WBCs]/µl), with a shift to the left of polymorphonuclear leukocytes (70% to 90% neutrophils) ▪ Urinalysis may show microscopic hematuria ▪ Erythrocyte sedimentation rate (ESR) elevated, especially in chronic or subacute bacterial endocarditis ▪ Prolonged partial thromboplastin time and prothrombin time; low fibrinogen and platelet counts, and low factor assays; possible disseminated intravascular coagulation ▪ Cultures of urine, sputum, and skin lesions with discharge may identify primary infection site; chest X-rays and scans of lungs, liver, abdomen, and brain may assist with identification ▪ Echocardiogram may show heart valve vegetation | ▪ Semisynthetic penicillins (oxacillin, nafcillin) or cephalosporins (cefazolin) given I.V. ▪ Vancomycin I.V. for patients with penicillin allergy or suspected methicillin-resistant organisms ▪ Possibly, probenecid given to partially prevent urinary excretion of penicillin and to prolong blood levels ▪ I.V. fluids to reverse shock ▪ Removal of infected catheter or foreign body ▪ Surgery | ▪ Report infection to authorities as required. ▪ S. aureus bacteremia can be fatal within 12 hours. Be especially alert for it in debilitated patients with I.V. catheters or in those with a history of drug abuse. ▪ Administer antibiotics on time to maintain adequate blood levels, but give them slowly, using the prescribed amount of diluent, to prevent thrombophlebitis. ▪ Watch for signs of penicillin allergy, especially pruritic rash (anaphylaxis) and breathing difficulties. Keep epinephrine ▪ 1:1,000 and resuscitation equipment handy. Monitor the patient’s vital signs, urine output, and mental state for signs of shock. ▪ Obtain cultures carefully, and observe for clues to the primary site of infection. Never refrigerate blood cultures; it delays identification of organisms by slowing their growth. ▪ If the patient has methicillin-resistant Staphylococcus aureus (MRSA): regardless of site, place patient on contact precautions. ▪ Obtain peak and trough levels of vancomycin to determine the adequacy of treatment. ▪ Administer vancomycin I.V. slowly over 1 hour to avoid any adverse reactions. |
Pneumonia | ||||
▪ Immune deficiencies, especially in elderly and in children younger than age 2 ▪ Chronic lung diseases and cystic fibrosis ▪ Malignant tumors ▪ Antibiotics that kill normal respiratory flora but spare S. aureus ▪ Viral respiratory infections, especially influenza ▪ Hematogenous (bloodborne) bacteria spread to the lungs from primary sites of infection (such as heart valves, abscesses, and pulmonary emboli) ▪ Recent bronchial or endotracheal suctioning or intubation | ▪ High temperature: adults, 103° to 105° F (39.4° to 40.6° C); children, 101° F (38.3° C) or above ▪ Cough, with purulent, yellow, or bloody sputum ▪ Dyspnea, crackles, and decreased breath sounds ▪ Pleuritic pain ▪ In infants: mild respiratory infection that suddenly worsens: irritability, anxiety, dyspnea, anorexia, vomiting, diarrhea, spasms of dry coughing, marked tachypnea, expiratory grunting, sternal retractions, and cyanosis ▪ Complications: necrosis, lung abscess, pyopneumothorax, empyema, pneumatocele, shock, hypotension, pleural effusions, respiratory failure, confusion | ▪ WBC count may be elevated (15,000 to 40,000/µl in adults; 15,000 to 20,000/µl in children), with predominance of polymorphonuclear leukocytes ▪ Sputum Gram stain: mostly gram-positive cocci in clusters, with many polymorphonuclear leukocytes ▪ Sputum culture: mostly coagulase-positive staphylococci ▪ Chest X-rays: usually patchy infiltrates ▪ Arterial blood gas analysis: hypoxia and respiratory acidosis | ▪ Semisynthetic penicillins (oxacillin, nafcillin) or cephalosporins (cefazolin) given I.V. ▪ Vancomycin I.V. for patients with penicillin allergy or suspected methicillin-resistant organisms ▪ Isolation for MRSA until the patient is off antibiotics and symptoms resolve (some facilities may require negative cultures) | ▪ The Centers for Disease Control and Prevention’s isolation guidelines require standard precautions unless MRSA, which requires contact precautions, is present. ▪ Keep the door to the patient’s room closed. Don’t store extra supplies in his room. Disposable suction containers are preferred. ▪ When obtaining sputum specimens, make sure you’re collecting thick sputum, not saliva. The presence of epithelial cells (found in the mouth, not lungs) indicates a poor specimen. ▪ Administer antibiotics strictly on time, but slowly. Watch for signs of penicillin allergy and for signs of infection at the I.V. sites. Change the I.V. site every third day. ▪ Perform frequent chest physical therapy. Do chest percussion and postural drainage after intermittent positive pressure breathing treatments. Concentrate on consolidated areas (revealed by X-rays or auscultation). |
Enterocolitis | ||||
▪ Broad-spectrum antibiotics (tetracycline, chloramphenicol, or neomycin) or aminoglycosides (tobramycin, streptomycin, or kanamycin) as prophylaxis for bowel surgery or treatment of hepatic coma ▪ Usually occurs in elderly patients, but also in neonates (associated with staphylococcal skin lesions) | ▪ Sudden onset of profuse, watery diarrhea usually 2 days to several weeks after start of antibiotic therapy, I.V. or by mouth (P.O.) ▪ Nausea, vomiting, abdominal pain and distention ▪ Hypovolemia and dehydration (decreased skin turgor, hypotension, fever) | ▪ Stool Gram stain: many grampositive cocci and polymorphonuclear leukocytes, with few gram-negative rods ▪ Stool culture: S. aureus ▪ Sigmoidoscopy: mucosal ulcerations ▪ Blood studies: leukocytosis, moderately increased blood urea nitrogen level, and decreased serum albumin level | ▪ Broad-spectrum antibiotics discontinued ▪ Possibly, antistaphylococcal agents such as vancomycin P.O. ▪ Normal flora replenished with yogurt that contains live cultures | ▪ Monitor vital signs frequently to detect early signs of shock. ▪ Force fluids to correct dehydration. ▪ Know serum electrolyte levels. Measure and record bowel movements when possible. Check serum chloride level for alkalosis (hypochloremia). Watch for dehydration and electrolyte imbalance. ▪ Collect serial stool specimens for Gram stain and culture to confirm diagnosis. (The effectiveness of therapy is usually measured by clinical response.) ▪ Observe standard precautions. Use contact precautions for diapered or incontinent children for duration of illness. ▪ Follow reporting requirements, especially in a group situation such as a nursing home. They may vary per facility protocol. |
Osteomyelitis | ||||
▪ Hematogenous organisms ▪ Skin trauma ▪ Infection spreading from adjacent joint or other infected tissues ▪ S. aureus bacteremia ▪ Orthopedic surgery or trauma ▪ Cardiothoracic surgery ▪ Usually occurs in growing bones, especially femur and tibia, of children younger than age 12 ▪ More common in males | ▪ Abrupt onset of fever—usually 101° F (38.3° C) or above; shaking chills; pain and swelling over infected area; restlessness; headache ▪ About 20% of children develop a chronic infection if not properly treated | ▪ Possible history of prior trauma to involved area ▪ Positive bone and pus cultures (and blood cultures in about 50% of patients) ▪ X-ray changes apparent after second or third week ▪ ESR elevated with leukocyte shift to the left | ▪ Surgical debridement ▪ Prolonged antibiotic therapy (4 to 8 weeks) ▪ Vancomycin I.V. for patients with penicillin allergy or methicillin-resistant organisms ▪ Possibly, removal of prosthesis or hardware | ▪ Identify the infected area, and mark it on the care plan. ▪ Check the penetration wound from which the organism originated for evidence of present infection. ▪ Severe pain may render the patient immobile. If so, perform passive range-of-motion exercises. Apply heat as needed, and elevate the affected part. (Extensive involvement may require casting until the infection subsides.) ▪ Before procedures such as surgical debridement, warn the patient to expect some pain. Explain that drainage is essential for healing, and that he will continue to receive analgesics and antibiotics after surgery. |
Food poisoning | ||||
▪ Enterotoxin produced by toxigenic strains of S. aureus in contaminated food (second most common cause of food poisoning in United States) | ▪ Anorexia, nausea, vomiting, diarrhea, and abdominal cramps 1 to 6 hours after ingestion of contaminated food ▪ Symptoms usually subside within 18 hours, with complete recovery occuring in 1 to 3 days | ▪ Clinical findings sufficient ▪ Stool cultures: usually negative for S. aureus ▪ Epidemiologic history: if others are ill and food history is a commonality; health department may be contacted for an outbreak | ▪ No treatment necessary unless dehydration becomes a problem (usually in infants and elderly); oral rehydrating solution or I.V. therapy may be necessary to replace fluids | ▪ Monitor vital signs, fluid balance, and serum electrolyte levels. ▪ Check for dehydration if vomiting is severe or prolonged and for decreased blood pressure. ▪ Observe and report the number and color of stools. ▪ Report infection to authorities as required. ▪ Obtain a complete history of symptoms, recent meals, and other known cases of food poisoning. |
Skin infections | ||||
▪ Decreased resistance ▪ Burns or pressure ulcers Decreased blood flow ▪ Possible skin contamination from nasal discharge ▪ Foreign bodies ▪ Underlying skin diseases, such as eczema and acne ▪ Common in people with poor hygiene living in crowded quarters ▪ Insulin-dependent diabetes mellitus ▪ Hemodialysis ▪ I.V. drug injection | ▪ Cellulitis—diffuse, acute inflammation of soft tissue (no discharge) ▪ Pus-producing lesions in and around hair follicles (folliculitis) ▪ Boil-like lesions (furuncles and carbuncles) extend from hair follicles to subcutaneous tissues; these painful, red, indurated lesions are 1 to 2 cm and have a purulent yellow discharge ▪ Small macules or skin blebs that may develop into vesicles containing pus (bullous impetigo); common in school-age children ▪ Mild or spiking fever ▪ Malaise | ▪ Clinical findings and analysis of pus cultures if sites are draining ▪ Cultures of nondraining cellulitis taken from the margin of the reddened area by infiltration with 1 mL sterile saline solution (nonbacteriostatic saline) and immediate fluid aspiration | ▪ Topical ointments; gentamicin or bacitracin-neomycin polymyxin ▪ P.O. cloxacillin, dicloxa cillin, or erythromycin; I.V. oxacillin or nafcillin for severe infection; I.V. vancomycin for methicillin-resistant organisms ▪ Application of heat to reduce pain ▪ Surgical drainage ▪ Identification and treatment of sources of reinfection (nostrils, perineum) ▪ Cleaning and covering the area with moist, sterile dressings | ▪ Identify the site and extent of infection. ▪ Keep lesions clean with saline solution and peroxide irrigations, as ordered. Cover infections near wounds or the genitourinary tract with gauze pads. Keep pressure off the site to facilitate healing. ▪ Be alert for the extension of skin infections. ▪ Severe infection or abscess may require surgical drainage. Explain the procedure to the patient. Determine if cultures will be taken, and be prepared to collect a specimen. ▪ Impetigo is contagious. Isolate the patient and alert his family. Use contact precautions for all draining lesions. ▪ Use contact precautions for duration of illness for major skin/wound infections where there is no dressing or dressing does not adequately contain drainage. |
Contact isolation precautions should be used when in contact with the patient. A disinfected private room should be made available with dedicated equipment.
Change gloves when contaminated or when moving from a “dirty” area of the body to a clean one.
Instruct the patient’s family and friends to wear protective clothing when they visit him, and show them how to dispose of it.
Provide teaching and emotional support to the patient and his family members.
Consider grouping infected patients together and having the same nursing staff care for them.
Don’t lay equipment used on the patient on the bed or bed stand. Be sure to wipe it with appropriate disinfectant before leaving the room.
Ensure judicious and careful use of antibiotics. Encourage physicians to limit their use.
Instruct the patient to take antibiotics for the full period prescribed, even if he begins to feel better.
the acute illness, but they may be evident after a nonsymptomatic illness.
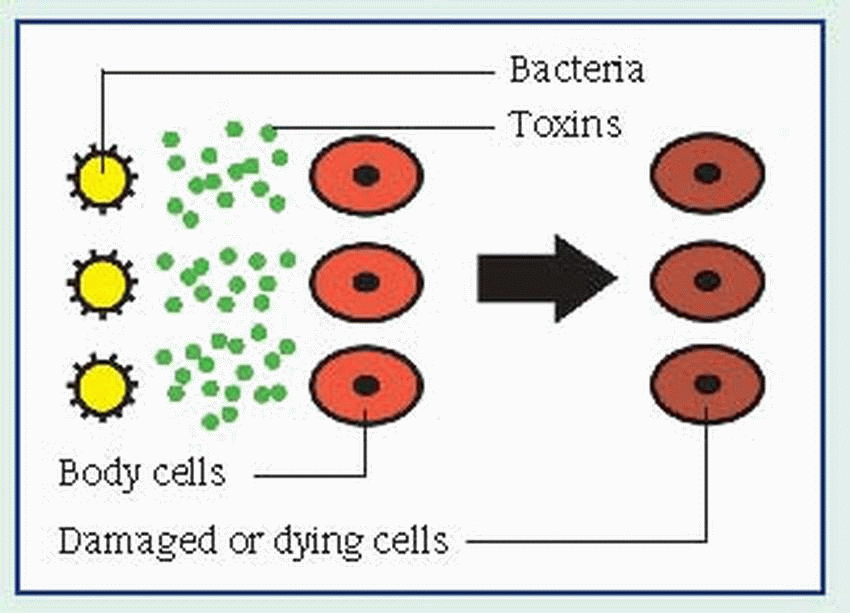
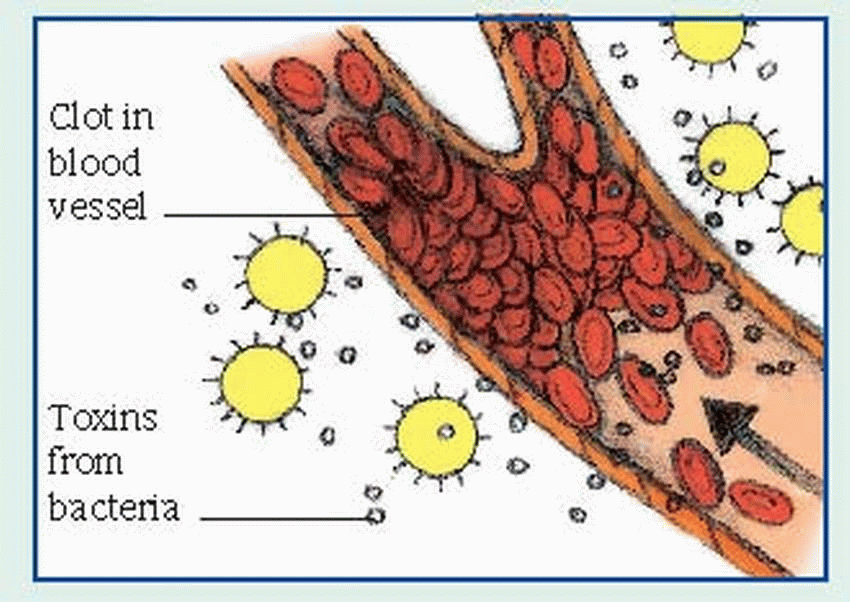
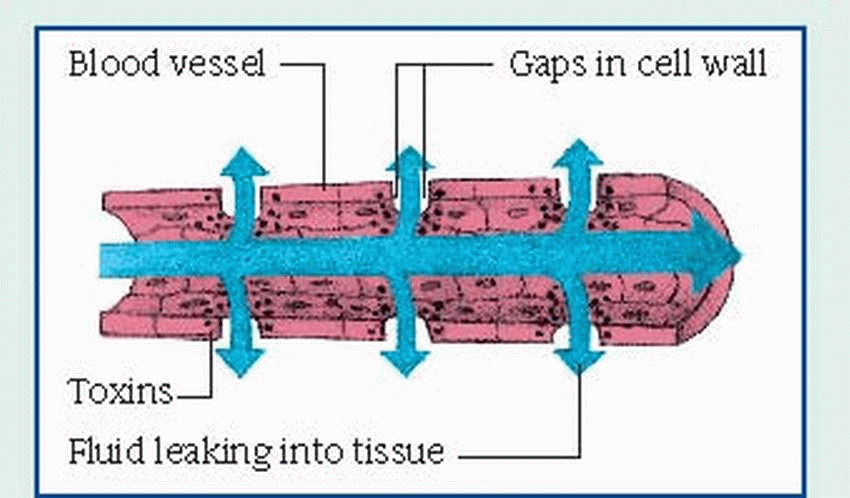
and Klebsiella, may be present. They can proliferate in an environment of tissue hypoxia caused by trauma, recent surgery, or medical compromise. The end product of this invasion is necrosis of the surrounding tissue, which accelerates the disease process by creating an even more favorable environment for the organisms.
Causes and incidence | Signs and symptoms | Diagnosis | Complications | Treatment and special considerations |
Streptococcus pyogenes (Group A streptococcus) | ||||
Streptococcal pharyngitis (strep throat) | ||||
▪ Accounts for 95% of all cases of bacterial pharyngitis ▪ Most common in children ages 5 to 10, from October to April ▪ Spread by direct person-to-person contact via droplets of saliva or nasal secretions ▪ Organism usually colonizes throats of persons with no symptoms ▪ Up to 20% of school children may be carriers | ▪ After 1- to 5-day incubation period: temperature of 101° to 104° F (38.3° to 40° C), sore throat with severe pain on swallowing, beefy red pharynx, tonsillar exudate, edematous tonsils and uvula, swollen glands along the jaw line, generalized malaise and weakness, anorexia, occasional abdominal discomfort ▪ Up to 40% of small children have symptoms too mild for diagnosis ▪ Fever abates in 3 to 5 days; nearly all symptoms subside within a week | ▪ Clinically indistinguishable from viral pharyngitis ▪ Throat culture showing group A beta-hemolytic streptococci (carriers have positive throat culture) ▪ Elevated white blood cell (WBC) count ▪ Serology showing a fourfold rise in streptozyme titers during convalescence | ▪ Acute otitis media or acute sinusitis occurs most frequently ▪ Rarely, bacteremic spread may cause arthritis, endocarditis, meningitis, osteomyelitis, or liver abscess ▪ Poststreptococcal sequelae: acute rheumatic fever or acute glomerulonephritis ▪ Reye’s syndrome | ▪ Penicillin or erythromycin, analgesics, and antipyretics may be ordered. ▪ Stress the need for bed rest and droplet precaution isolation from other children for 24 hours after antibiotic therapy begins; the patient should finish his prescription, even if symptoms subside; abscess, glomerulonephritis, and rheumatic fever can occur. ▪ Tell the patient not to skip doses and to properly dispose of soiled tissues. |
Scarlet fever (scarlatina) | ||||
▪ Usually follows streptococcal pharyngitis; may follow wound infections or puerperal sepsis ▪ Caused by streptococcal strain that releases an erythrogenic toxin ▪ Most common in children ages 2 to 10 ▪ Spread by large respiratory droplets or direct contact with items soiled with respiratory secretions | ▪ Streptococcal sore throat, fever, strawberry tongue, fine erythematous rash that blanches on pressure and resembles sunburn with goosebumps ▪ Rash usually appearing first on upper chest, then spreading to neck, abdomen, legs, and arms, sparing soles and palms; flushed cheeks, pallor around mouth ▪ Skin sheds during convalescence | ▪ Characteristic rash and strawberry tongue ▪ Culture and Gram stain showing S. pyogenes from nasopharynx ▪ Granulocytosis | ▪ Although rare, complications may include high fever, arthritis, jaundice, pneumonia, pericarditis, and peritonsillar abscess | ▪ Penicillin or erythromycin may be ordered. ▪ Keep the patient in isolation for the first 24 hours. ▪ Carefully dispose of purulent discharge. ▪ Stress the need for prompt and complete antibiotic treatment. |
Erysipelas | ||||
▪ Occurs primarily in infants and adults older than age 30 ▪ Usually follows streptococcal pharyngitis ▪ Exact mode of spread to skin unknown | ▪ Sudden onset, with reddened, swollen, raised lesions (skin looks like an orange peel), usually on face and scalp, bordered by areas that often contain easily ruptured blebs filled with yellow-tinged fluid; lesions sting and itch; lesions on the trunk, arms, or legs usually affect incision or wound sites ▪ Other symptoms: vomiting, fever, headache, cervical lymphadenopathy, sore throat | ▪ Typical reddened lesions ▪ Culture taken from edge of lesions showing group A beta-hemolytic streptococci ▪ Throat culture almost always positive for group A beta-hemolytic streptococci | ▪ Untreated lesions on trunk, arms, or legs may involve large body areas and lead to death | ▪ Penicillin or erythromycin I.V. or by mouth (P.O.) may be ordered. ▪ Cold packs, analgesics (aspirin and codeine for local discomfort), and topical anesthetics may be used to increase comfort. ▪ Prevention includes prompt treatment of streptococcal infections and drainage and secretion precautions. |
Impetigo (streptococcal pyoderma) | ||||
▪ Common in children ages 2 to S in hot, humid weather; high rate of familial spread ▪ Predisposing factors: close contact in schools, overcrowded living quarters, poor skin hygiene, minor skin trauma ▪ May spread by direct contact, environmental contamination, or arthropod vector | ▪ Small macules rapidly develop into vesicles, then become pustular and encrusted, causing pain, surrounding erythema, regional adenitis, cellulitis, and itching; scratching spreads infection ▪ Lesions commonly affect the face, heal slowly, and leave depigmented areas | ▪ Characteristic lesions with honey-colored crust ▪ Culture and Gram stain of swabbed lesions showing S. pyogenes | ▪ Septicemia (rare) ▪ Ecthyma, a form of impetigo with deep ulcers | ▪ Penicillin I.V. or P.O., erythromycin, or antibiotic ointments may be ordered. ▪ Perform frequent washing of lesions with antiseptics, such as povidone-iodine or antibacterial soap, followed by thorough drying. ▪ Isolate a patient with draining wounds, using contact precautions. ▪ Prevention includes good hygiene and proper wound care. |
Streptococcus agalactiae (Group B streptococcus) | ||||
Neonatal streptococcal infections | ||||
▪ Incidence of early-onset infection (age 6 days or younger): 2/1,000 live births ▪ Incidence of late-onset infection (age 7 days to 3 months): 1/1,000 live births ▪ Spread by vaginal delivery or hands of nursery staff ▪ Predisposing factors: maternal genital tract colonization, membrane rupture over 24 hours before delivery, crowded nursery | ▪ Early onset: bacteremia, pneumonia, and meningitis; mortality from 14% for infants weighing more than 1,500 g at birth to 61% for infants weighing less than 1,500 g at birth ▪ Late onset: bacteremia with meningitis, fever, and bone and joint involvement; mortality 15% to 20% ▪ Other signs and symptoms, such as skin lesions, depend on the site affected | ▪ Isolation of group B streptococcus from blood, cerebrospinal fluid (CSF), or skin ▪ Chest X-ray showing massive infiltrate similar to that of respiratory distress syndrome or pneumonia | ▪ Overwhelming pneumonia, sepsis, and death | ▪ Penicillin or ampicillin and an aminoglycoside I.V. may be ordered. ▪ Patient isolation is unnecessary unless an open draining lesion is present, but proper hand-hygiene is essential; for a draining lesion, take drainage and secretion precautions. ▪ Group B streptococcus prophylaxis may be ordered for women who are pregnant if vaginal or rectal cultures are positive at 35 to 37 weeks’ gestation or if the patient meets other criteria, such as delivery earlier or later than 3 weeks of term, amniotic fluid rupture for 18 hours or more, or an intrapartum temperature greater than or equal to 100.4° F [38.0° C]). |
Adult group B streptococcal infection | ||||
▪ Most adult infections occur in postpartum women, usually in the form of endometritis or wound infection following cesarean section ▪ Incidence of group B streptococcal endometritis: 1.3/1,000 live births ▪ Group B streptococcal bacteremia and pneumonia: occur in the elderly and frequently in patients with diabetes ▪ Invasive group B streptococcal infection: occurs in patients with human immunodeficiency virus | ▪ Fever, malaise, and uterine tenderness ▪ Change in lochia ▪ Bacteremia and pneumonia patients: can exhibit neurologic symptoms such as a change in mental status | ▪ Isolation of group B streptococcus from blood or infection site | ▪ Bacteremia followed by meningitis or endocarditis | ▪ Ampicillin or penicillin I.V. may be ordered. ▪ Perform careful observation for symptoms of infection following delivery. ▪ Follow drainage and secretion precautions. |
Streptococcus pneumoniae | ||||
Pneumococcal pneumonia | ||||
▪ Accounts for 70% of all cases of bacterial pneumonia ▪ More common in men, elderly, Blacks, and Native Americans, in winter and early spring ▪ Spread by droplets and contact with infective secretions ▪ Predisposing factors: trauma, viral infection, underlying pulmonary disease, overcrowded living quarters, chronic diseases, asplenia, and immunodeficiency ▪ Among the 10 leading causes of death in the United States | ▪ Sudden onset with severe shaking chills, temperature of 102° to 105° F (38.9° to 40.6° C), bacteremia, cough (with thick, scanty, blood-tinged sputum) accompanied by pleuritic pain ▪ Malaise, weakness, and prostration common ▪ Tachypnea, anorexia, nausea, and vomiting less common ▪ Severity of pneumonia usually caused by host’s cellular defenses, not bacterial virulence | ▪ Gram stain of sputum showing gram-positive diplococci; culture showing S. pneumoniae ▪ Chest X-ray showing lobular consolidation in adults; bronchopneumonia in children and elderly patient ▪ Elevated WBC count ▪ Blood cultures usually positive for S. pneumoniae | ▪ Pleural effusion (occurs in 25% of patients) ▪ Pericarditis (rare) ▪ Lung abscess (rare) ▪ Bacteremia ▪ Disseminated intravascular coagulation ▪ Death possible if bacteremia is present | ▪ Penicillin or erythromycin I.V. or I.M. may be ordered. ▪ Monitor and support respirations, as needed. ▪ Record sputum color and amount. ▪ Prevent dehydration. ▪ Avoid sedatives and opioids to preserve cough reflex. ▪ Carefully dispose of all purulent drainage (standard precautions); advise high-risk patients to receive a vaccine and to avoid infected people. |
Otitis media | ||||
▪ High incidence, with about 76% to 95% of all children having otitis media at least once (S. pneumoniae causes half of these cases.) | ▪ Ear pain, ear drainage, hearing loss, fever, lethargy, and irritability ▪ Other possible symptoms: vertigo, nystagmus, and tinnitus | ▪ Fluid in middle ear ▪ Isolation of S. pneumoniae from aspirated fluid if necessary | ▪ Recurrent attacks (may cause hearing loss) | ▪ Amoxicillin or ampicillin and analgesics may be ordered. ▪ Tell the patient to report a lack of response to therapy after 72 hours. |
Meningitis | ||||
▪ Can follow bacteremic pneumonia, mastoiditis, sinusitis, skull fracture, or endocarditis ▪ Mortality (30% to 60%) highest in infants and elderly people | ▪ Fever, headache, nuchal rigidity, vomiting, photophobia, lethargy, coma, wide pulse pressure, and bradycardia | ▪ Isolation of S. pneumoniae from CSF or blood culture ▪ Increased CSF cell count and protein level; decreased CSF glucose level ▪ Computed tomography scan of head ▪ EEG | ▪ Persistent hearing deficits, seizures, hemiparesis, or other nerve deficits ▪ Encephalitis | ▪ Penicillin I.V. or chloramphenicol may be ordered. ▪ Monitor the patient closely for neurologic changes. ▪ Watch for symptoms of septic shock, such as acidosis and tissue hypoxia. |
Group D streptococcus | ||||
Endocarditis | ||||
▪ Group D streptococcus (enterococcus): causes 10% to 20% of all bacterial endocarditis ▪ Most common in elderly people and in those who abuse I.V. substances ▪ Typically follows bacteremia from an obvious source, such as a wound infection, urinary tract infection, or I.V. insertion site infection ▪ Most cases are subacute ▪ Also causes urinary tract infection | ▪ Weakness, fatigability, weight loss, fever, night sweats, anorexia, arthralgia, splenomegaly, and new systolic murmur | ▪ Anemia, increased erythrocyte sedimentation rate and serum immuno-globulin level, and positive blood culture for group D streptococcus ▪ Echocardiogram showing vegetation on valves | ▪ Embolization ▪ Pulmonary infarction ▪ Osteomyelitis | ▪ Penicillin for Streptococcus bovis (non-enterococcal group D streptococcus) may be ordered. ▪ Penicillin or ampicillin and an aminoglycoside for enterococcal group D streptococcus may be ordered. |
Renal failure
Septic shock
Cardiovascular collapse
Scarring
Myosis
Myonecrosis
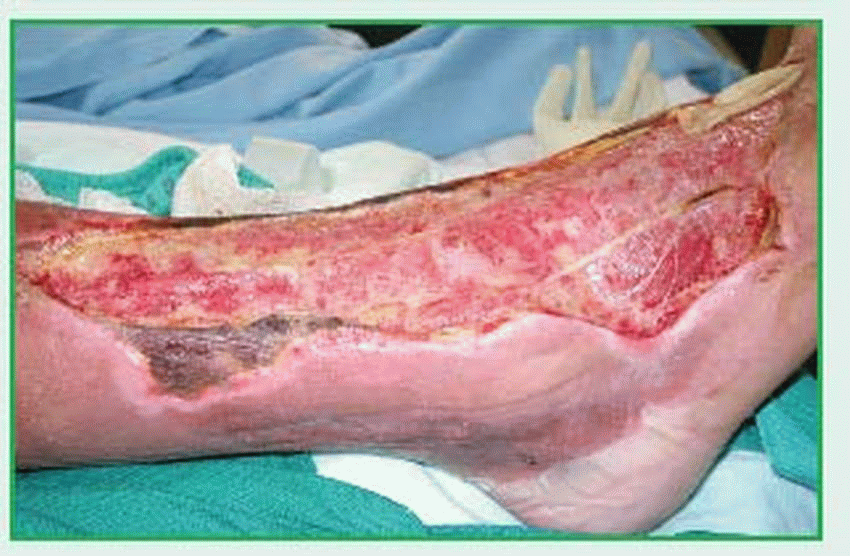
the rapid progression of the necrotizing process. By days 4 and 5, multiple patches of this erythema form, producing large areas of gangrenous skin. By days 7 to 10, dead skin begins to separate at the margins of the erythema, revealing extensive necrosis of the subcutaneous tissue. At this stage, fascial necrosis is typically more advanced than appearance would suggest.
organisms and the effective treatment against them.
Antibiotic therapy should be initiated immediately.
Accurate and frequent assessment of the patient’s pain level, mental status, wound status, and vital signs is essential in order to recognize the progression of the wound changes or the development of new signs and symptoms. Changes must be reported and documented immediately.
The need for supportive care, such as endotracheal intubation, cardiac monitoring, fluid replacement, and supplemental oxygen, should be assessed and provided as warranted.
Care of postoperative patients and patients with trauma wounds requires strict sterile technique, good hand hygiene, and barriers between health care providers and patients to prevent contamination.
Use contact precautions for draining wounds for 24 hours after beginning appropriate antibiotic therapy. Use droplet precautions, especially for bedside wound debridement. Outbreaks of serious invasive disease have occurred secondary to transmission among patients and health care workers.
Health care workers with sore throats should see their physician to determine if they have a streptococcal infection. If they are diagnosed positive, they shouldn’t return to work until 24 hours after the initiation of antibiotic therapy.
Risk factors for contracting necrotizing fasciitis include patients with advanced age, human immunodeficiency virus infection, history of alcohol abuse, and varicellar infection. Patients with chronic illnesses, such as cancer, diabetes, cardiopulmonary disease, and kidney disease requiring hemodialysis, as well as those using steroids are more susceptible to GAS infection due to their debilitated immune response.
Wash your hands before and after providing patient care. Good hand washing is the most effective way to prevent VISA and VRSA from spreading. Use an antiseptic soap such as chlorhexidine; bacteria have been cultured from workers’ hands after washing with milder soap.
Minimize the number of staff caring for the patient.
Contact isolation precautions should be used when in contact with the patient. A private room should be used. Use dedicated equipment and disinfect the environment.
Change gloves when contaminated or when moving from a soiled area of the body to a clean one.
Wear mask/eye protection or a face shield when performing splash-generating activities, such as suctioning.
Don’t touch potentially contaminated surfaces, such as a bed or bed stand, after removing gown and gloves.
Be particularly cautious in caring for a patient with an ileostomy, colostomy, or draining wound that isn’t contained by a dressing.
Equipment used on the patient shouldn’t be laid on the bed or bed stand and should be wiped with appropriate disinfectant before leaving the room.
Ensure judicious and careful use of antibiotics. Encourage physicians to limit the use of antibiotics.
Instruct family and friends to wear protective garb when they visit the patient. Demonstrate how to dispose of it.
Provide teaching and emotional support to the patient and his family members.
Instruct the patient to take antibiotics for the full prescription period, even if he begins to feel better.
Contact public health authorities before transfer or discharge.
immunosuppressed patients or those with severe underlying disease
patients with a history of taking vancomycin, third-generation cephalosporins, antibiotics targeted at anaerobic bacteria (such as Clostridium difficile), or multiple courses of antibiotics
patients with indwelling urinary or central venous catheters
elderly patients, especially those with prolonged or repeated hospital admissions
patients with cancer or chronic renal failure
patients undergoing cardiothoracic or intraabdominal surgery or organ transplant
patients with wounds opening into the pelvic or intra-abdominal area, including surgical wounds, burns, and pressure ulcers
patients with enterococcal bacteremia, typically associated with endocarditis
patients exposed to contaminated equipment or to another VRE-positive patient
Sepsis
Multisystem dysfunction
Pneumonia
Meningitis
Endocarditis
Death (in immunocompromised patients)
VRE strain. Combinations of various drugs may also be used, depending on the source of the infection.
Hand hygiene before and after care of the patient is crucial. Good hand hygiene is the most effective way to prevent VRE from spreading. Use an antiseptic soap such as chlorhexidine. Bacteria have been cultured from workers’ hands after they’ve washed with milder soap. Alcohol-based hand sanitizers are effective as well.
Use contact precautions when in contact with the patient or his support equipment. Provide the patient with a private room and dedicated equipment. Disinfect the environment and the equipment frequently.
Change gloves when contaminated or when moving from a “dirty” area of the body to a clean one.
Don’t touch potentially contaminated surfaces such as an overbed table after removing your gown and gloves.
Be particularly prudent in caring for a patient with an ileostomy, colostomy, or draining wound that isn’t contained by a dressing.
Instruct the patient’s family and friends to wear protective garb when they visit him, and teach them how to dispose of it. Instruct them on proper hand hygiene.
Provide teaching and emotional support to the patient and his family members.
Consider grouping (“cohorting”) infected or colonized patients together and assigning the same nursing staff to them.
Don’t lay equipment used on the patient on the bed or on the overbed table. Wipe the equipment with the appropriate disinfectant before leaving the room.
Ensure judicious and careful use of antibiotics. Encourage physicians to limit their use.
Instruct patients to take antibiotics for the full period prescribed, even if they begin to feel better.
Report to public health authorities.
Infection of the middle ear
Pneumonia
Acute rheumatic fever
Hepatitis
Glomerulonephritis
Implement droplet precautions for 24 hours after starting antibiotic therapy.
Keep the patient on complete bed rest while he’s febrile to prevent complications, promote recovery, and help conserve his energy.
Offer frequent oral fluids and oral hygiene and give antipyretics as ordered.
Apply topical anesthetics on the patient’s tongue and throat to relieve pain.
Provide skin care to relieve discomfort from the rash.
Instruct the patient (or his parents) to make sure he takes his oral antibiotics for the prescribed length of time.
Respiratory failure
Disseminated intravascular coagulation (DIC)
Septic arthritis
Pericarditis
Endophthalmitis
Neurologic deterioration
Death
Give I.V. antibiotics, as ordered, to maintain blood and CSF drug levels.
Enforce bed rest in early stages. Provide a dark, quiet, restful environment.
Maintain adequate ventilation with oxygen or a ventilator, if necessary. Suction and turn the patient frequently.
Keep accurate intake and output records to maintain proper fluid and electrolyte levels. Monitor blood pressure, pulse, arterial blood gas levels, and CVP.
Watch for complications, such as DIC, arthritis, endocarditis, and pneumonia.
If the patient is receiving chloramphenicol, monitor complete blood count.
Check the patient’s drug history for allergies before giving antibiotics.
Impose droplet precautions until the patient has had antibiotic therapy for 24 hours.
Label all meningococcal specimens. Deliver them to the laboratory quickly because meningococci are very sensitive to changes in humidity and temperature.
Report all meningococcal infections to public health department officials.
To prevent the spread of this disease, stress the need for droplet precautions. Teach proper disposal of nasopharyngeal secretions. Maintain infection precautions until after two consecutive negative nasopharyngeal cultures—at least 1 week after discontinuing drug therapy. Treatment of exposed individuals with antitoxin remains controversial. Suggest that the patient’s family receive diphtheria toxoid if they haven’t been immunized.
Give drugs as ordered. Although timeconsuming and risky, desensitization should be attempted if tests are positive, because diphtheria antitoxin is the only specific treatment available. If sensitivity tests are negative, the antitoxin is given before laboratory confirmation, because mortality increases directly with any delay in antitoxin administration. Before giving diphtheria antitoxin, which is made from horse serum, obtain eye and skin tests to determine sensitivity. After giving antitoxin or penicillin, be alert for anaphylaxis; keep epinephrine 1:1,000 and resuscitation equipment handy. In patients who receive erythromycin, watch for thrombophlebitis.
Monitor respirations carefully, especially in laryngeal diphtheria (usually, such patients are in a high-humidity environment). Watch for signs of airway obstruction, and be ready to give immediate life support, including intubation and tracheotomy.
Watch for signs of shock, which can develop suddenly.
Obtain cultures as ordered.
If neuritis develops, tell the patient it’s usually transient. Be aware that peripheral neuritis may not develop until 2 to 3 months after the onset of illness.
Report all cases to public health authorities.
Sepsis
Diffuse clotting dyscrasias
Respiratory insufficiency
Circulatory insufficiency
Meningitis
Cerebritis
Nonpurulent conjunctivitis
Granulomatous skin infection
Long-term neurologic damage and delayed development in infants
a febrile, generalized illness. In a pregnant woman, especially during the third trimester, listeriosis causes a mild illness with malaise, chills, fever, and back pain. However, a severe uterine infection may produce abortion, premature delivery, or stillbirth. Transplacental infection may also cause early neonatal death or granulomatosis infantiseptica, which produces organ abscesses in infants.
Deliver specimens to the laboratory promptly. Because few organisms may be present, take at least 10 ml of spinal fluid for culture.
Use standard precautions until a series of cultures are negative. Be especially careful when handling lochia from an infected mother and secretions from her infant’s eyes, nose, mouth, and rectum, including meconium.
Evaluate neurologic status at least every 2 hours. In an infant, check fontanels for bulging. Maintain adequate I.V. fluid intake; measure intake and output accurately.
If the patient has central nervous system depression and becomes apneic, provide respiratory assistance, monitor respirations, and obtain frequent arterial blood gas measurements.
Provide adequate nutrition by total parenteral nutrition, nasogastric tube feedings, or a soft diet, as ordered.
Allow the patient’s parents to see and, if possible, hold their infant in the neonatal intensive care unit. Be flexible about visiting privileges. Keep the parents informed of the infant’s status and prognosis at all times.
Reassure the parents of an infected neonate who may feel guilty about the infant’s illness.
Report all cases to public health authorities.
To avoid infection, instruct the patient and his family to avoid soft cheeses and to cook such foods as hot dogs thoroughly. Immunocompromised patients should avoid soft cheeses and deli meats.
Atelectasis
Pneumonia
Pulmonary emboli
Acute gastric ulcers
Seizures
Flexion contractures
Cardiac arrhythmias
neck and facial muscles, especially cheek muscles—locked jaw (trismus), painful spasms of masticatory muscles, difficulty opening the mouth, and risus sardonicus, a grotesque, grinning expression produced by spasm of facial muscles
somatic muscles—arched-back rigidity (opisthotonos); boardlike abdominal rigidity
intermittent tonic seizures lasting several minutes, which may result in cyanosis and sudden death by asphyxiation
Thoroughly debride and clean the injury site, and check the patient’s immunization history. Record the cause of injury. If it’s an animal bite, report the case to local public health authorities.
Before giving penicillin and TIG, antitoxin, or toxoid, obtain an accurate history of allergies to immunizations or penicillin. If the patient has a history of allergies, keep epinephrine 1:1,000 and resuscitation equipment available.
Stress the importance of maintaining active immunization with a booster dose of tetanus toxoid every 10 years.
Maintain an adequate airway and ventilation to prevent pneumonia and atelectasis. Suction often and watch for signs of respiratory distress. Keep emergency airway equipment on hand because the patient may require artificial ventilation or oxygen administration.
Maintain an I.V. line for medications and emergency care, if necessary.
Monitor the electrocardiogram frequently for arrhythmias. Record intake and output accurately, and check vital signs often.
Turn the patient frequently to prevent pressure ulcers and pulmonary stasis.
Because even minimal external stimulation provokes muscle spasms, keep the patient’s room quiet and only dimly lighted. Warn visitors not to upset or overly stimulate the patient.
If urine retention develops, insert an indwelling urinary catheter.
Give muscle relaxants and sedatives, as ordered, and schedule patient care—such as passive range-of-motion exercises—to coincide with periods of heaviest sedation.
Rinse the wound thoroughly with clean water.
Clean the wound and the area around it with soap and a washcloth.
Contact a practitioner if debris is embedded in the wound.
Tell the patient to contact a practitioner if the wound is deep, especially if it’s dirty or is a result of an animal bite.
Tell the patient to contact a practitioner if the date of his most recent tetanus shot is uncertain.
Insert an artificial airway, if necessary, to prevent tongue injury and maintain airway during spasms.
Provide adequate nutrition to meet the patient’s increased metabolic needs. The patient may need nasogastric feedings or total parenteral nutrition. (See Preventing tetanus.)
of contaminated food. Severity varies with the amount of toxin ingested and the patient’s degree of immunocompetence. Generally, early onset (within 24 hours) signals critical and potentially fatal illness. Initial signs and symptoms include dry mouth, sore throat, weakness, dizziness, vomiting, and diarrhea. The cardinal sign of botulism, though, is acute symmetrical cranial nerve impairment (ptosis, diplopia, and dysarthria), followed by descending weakness or paralysis of muscles in the extremities or trunk, and dyspnea from respiratory muscle paralysis. Such impairment doesn’t affect mental or sensory processes and isn’t associated with fever.
Obtain a careful history of the patient’s food intake for the past several days. See if other family members exhibit similar symptoms and share a common food history.
Observe carefully for abnormal neurologic signs. Tell the patient’s family to watch for signs of weakness, blurred vision, and slurred speech after he has returned home. If such signs appear, the patient must return to the hospital immediately.
If ingestion has occurred within several hours, induce vomiting, begin gastric lavage, and give a high enema to purge any unabsorbed toxin from the bowel.
Admit the patient to the intensive care unit, and monitor cardiac and respiratory functions carefully.
Administer botulinum antitoxin, as ordered, to neutralize any circulating toxin. Before giving the antitoxin, be sure to obtain an accurate patient history of allergies, especially to horses, and perform a skin test. Afterward, watch for anaphylaxis or other hypersensitivity and serum sickness. Keep epinephrine 1:1,000 (for subcutaneous administration) and emergency airway equipment available.
Assess respiratory function every 4 hours. Report decreased vital capacity on inspiratory effort and any signs of respiratory distress.
Closely assess and accurately record neurologic function, including bilateral motor status (reflexes, ability to move arms and legs).
Give I.V. fluids as ordered. Turn the patient often, and encourage deep-breathing exercises. Isolation isn’t required.
As botulism is sometimes fatal, keep the patient and his family informed regarding the course of the disease.
Immediately report all cases of botulism to public health authorities.
species). It occurs in devitalized tissues and results from compromised arterial circulation after trauma, surgery, compound fractures, or lacerations. This rare infection carries a high mortality unless therapy begins immediately; however, with prompt treatment, 80% of patients with gas gangrene of the extremities survive. The prognosis is poorer for gas gangrene in other sites, such as the abdominal wall or the bowel. The usual incubation period is 1 to 4 days but can vary from 3 hours to 6 weeks or longer.
Renal failure
Shock
Hemolytic anemia
Jaundice with liver damage
Tissue death
Amputation
(cool skin; pallor or cyanosis; sudden, severe pain; sudden edema; and loss of pulses in involved limb).
Throughout this illness, provide adequate fluid replacement, and assess pulmonary and cardiac functions often. Maintain airway and ventilation.
To prevent skin breakdown and further infection, give good skin care. After surgery, provide meticulous wound care. Use contact precautions for significant drainage.
Before penicillin administration, obtain a patient history of allergies; afterward, watch closely for signs of hypersensitivity.
Psychological support is critical, because these patients can remain alert until death, knowing that death is imminent and unavoidable.
Deodorize the room to control foul odor from the wound. Prepare the patient emotionally for a large wound after surgical excision, and refer him for physical rehabilitation, as necessary.
Institute standard precautions. Use contact precautions if drainage is significant. Dispose of drainage material properly, and wear sterile gloves when changing dressings. Spore-forming bacteria aren’t destroyed by ordinary disinfecting methods. Contaminated items should be cleaned and disinfected or sterilized, as appropriate.
Be alert for devitalized tissues, and notify the surgeon promptly.
Position the patient to facilitate drainage, and eliminate all dead spaces in closed wounds.
Sinus and maxilla facial subcutaneous tissue involvement
Abscesses and fistulas of the brain
Pneumonia
Empyema
microscopic examination of sulfur granules Gram staining of excised tissue or exudate to reveal branching gram-positive rods
chest X-ray to show lesions in unusual locations such as the shaft of a rib
Dispose of all dressings in a sealed plastic bag.
After surgery, provide proper sterile wound management.
Administer antibiotics as ordered. Before giving the first dose, obtain an accurate patient history of allergies. Watch for hypersensitivity reactions, such as rash, fever, itching, and signs of anaphylaxis. If the patient has a history of any allergies, keep epinephrine 1:1,000 and resuscitation equipment available.
Meningitis
Seizures
Cardiac arrhythmias
Provide adequate nourishment through total parenteral nutrition, nasogastric tube feedings, or a balanced diet.
Give the patient tepid sponge baths and antipyretics, as ordered, to reduce his fever.
Monitor for allergic reactions to antibiotics.
High-dose sulfonamide therapy (especially sulfadiazine) predisposes the patient to crystalluria and oliguria; so assess him frequently, force fluids, and alkalinize the urine with sodium bicarbonate, as ordered, to prevent these complications.
In patients with pulmonary infection, administer chest physiotherapy. Auscultate the lungs daily, checking for increased crackles or consolidation. Note and record the amount, color, and thickness of sputum.
In brain infection, regularly assess neurologic function. Watch for signs of increased intracranial pressure, such as a decreased level of consciousness and respiratory abnormalities.
In long-term hospitalization, turn the patient often, and assist with range-of-motion exercises.
Before the patient is discharged, stress the need to follow a regular medication schedule to maintain therapeutic blood levels and to continue drugs even after symptoms subside. Explain the importance of frequent follow-up examinations.
Provide support and encouragement to help the patient and his family cope with this longterm illness.
Electrolyte abnormalities
Hypovolemic shock
Anasarca (caused by hypoalbuminemia)
Toxic megacolon
Colonic perforation
Peritonitis
Sepsis
Hemorrhage
Death (rare)
cell cytotoxin test—the gold standard for diagnosis of C. difficile; it tests for both toxin A and B; this takes 2 days to perform. It’s highly sensitive and specific for C. difficile.
molecular tests—the Food and Drug Administration has approved polymerase chain reation (PCR) assay test for the gene encoding toxin B.
enzyme immunoassays—slightly less sensitive than the cell cytotoxin test but has a turnaround time of only a few hours. Specificity is excellent.
stool culture—the most sensitive test; has a turnaround time of 2 days to obtain results. Non-toxin-producing strains of C. difficile can be easily identified; discovery of the toxin in stool requires further testing.
endoscopy (flexible sigmoidoscopy)—may be used in a patient who presents with an acute abdomen but no diarrhea, making it difficult to obtain a stool specimen. If pseudomembranes are visualized, treatment for C. difficile is usually initiated.
are mildly symptomatic. This is usually the only treatment needed. In more severe cases, metronidazole or vancomycin are effective therapies; metronidazole is the preferred treatment. Retesting for C. difficile is unnecessary if symptoms resolve.
Patients with known or suspected C. difficile diarrhea who are unable to practice good hygiene should be placed on contact precautions in a single room or in a room with other patients with similar status.
Use contact precautions for contact with blood and body fluids and for all direct contact with the patient and his immediate environment.
Wash your hands with an antiseptic soap after direct contact with the patient or the immediate environment. Alcohol hand rubs will not inactivate C. difficile spores.
A patient who is asymptomatic, without diarrhea or fecal incontinence for 72 hours, and who is able to practice good hygiene may have contact precautions discontinued.
Make sure reusable equipment is disinfected before it’s used on another patient.
Teach good hand-washing technique to prevent the spread of the infection.
Review proper disinfection of contaminated clothing or household items.
Tell the patient to inform health care workers of his condition before admission.
typhoid patients are younger than age 30; most carriers are women older than age 50. Incidence of typhoid in the United States is increasing as a result of travelers returning from endemic areas.
Type | Cause | Clinical features |
Bacteremia | Any Salmonella species, but most commonly S. choleraesuis. Incubation period: variable | Fever, chills, anorexia, weight loss (without GI symptoms), and joint pain |
Enterocolitis | Any species of nontyphoidal Salmonella, but usually S. enteritidis. Incubation period: 6 to 48 hours | Mild to severe abdominal pain, diarrhea, sudden fever of up to 102° F (38.9° C), nausea, and vomiting; usually self-limiting, but may progress to enteric fever (resembling typhoid), local abscesses (usually abdominal), dehydration, and septicemia |
Localized infections | Usually follows bacteremia caused by Salmonella species | Site of localization determines symptoms; localized abscesses may cause osteomyelitis, endocarditis, bronchopneumonia, pyelonephritis, and arthritis |
Paratyphoid | S. paratyphi and S. schottmuelleri (formerly S. paratyphi B). Incubation period: 3 weeks or more | Fever and transient diarrhea; generally resembles typhoid but less severe |
Typhoid fever | S. typhi enters the GI tract and invades the bloodstream via the lymphatics, setting up intracellular sites. During this phase, infection of the biliary tract leads to intestinal seeding with millions of bacilli. Involved lymphoid tissues (especially Peyer’s patches in the ilium) enlarge, ulcerate, and necrose, resulting in hemorrhage. Incubation period: usually 1 to 2 weeks | Symptoms of enterocolitis may develop within hours of ingestion of S. typhi; they usually subside before onset of typhoid fever symptoms First week: gradually increasing fever, anorexia, myalgia, malaise, headache, and slow pulse Second week: remittent fever up to 104° F (40° C) usually in the evening, chills, diaphoresis, weakness, delirium, increasing abdominal pain and distention, diarrhea or constipation, cough, moist crackles, tender abdomen with enlarged spleen, and maculopapular rach (especially on abdomen) Third week: persistent fever, increasing fatigue and weakness; usually subsides by end of third week, although relapses may occur Complications: intestinal perforation or hemorrhage, abscesses, thrombophlebitis, cerebral thrombosis, pneumonia, osteomyelitis, myocarditis, acute circulatory failure, and chronic carrier state |
Intestinal perforation
Intestinal hemorrhage
Cerebral thrombosis
Pneumonia
Endocarditis
Myocarditis
Meningitis
Pyelonephritis
Osteomyelitis
Cholecystitis
Hepatitis
Septicemia
Acute circulatory failure
Reactive arthritis
Explain the causes of salmonella infection.
Show the patient how to wash his hands by wetting them under running water, lathering with soap and scrubbing, rinsing under running water with his fingers pointing down, and drying with a clean towel or paper towel.
Tell the patient to wash his hands after using the bathroom and before eating.
Tell the patient to cook foods thoroughly— especially eggs and chicken—and to refrigerate them at once.
Teach the patient how to avoid crosscontaminating foods by cleaning preparation surfaces with hot, soapy water and drying them thoroughly after use; cleaning surfaces between foods when preparing more than one food; and washing his hands before and after handling each food.
Tell the patient with a positive stool culture to avoid handling food and to use a separate bathroom or clean the bathroom after each use.
Tell the patient to report dehydration, bleeding, or recurrence of signs of salmonella infection.
All infections caused by Salmonella must be reported to the state health department.
Follow contact precautions if the patient is incontinent or diapered; otherwise, standard precautions are appropriate. Always wash your hands thoroughly before and after any contact with the patient, and advise other facility personnel to do the same. Teach the patient to use proper hand washing, especially after defecating and before eating or handling food. Wear gloves and a gown when disposing of feces or fecally contaminated objects. Continue precautions until three consecutive stool cultures are negative—the first one taken 48 hours after antibiotic treatment ends, followed by two more at 24-hour intervals.
Observe the patient closely for signs and symptoms of bowel perforation from erosion of intestinal ulcers: sudden pain in the lower right side of the abdomen and abdominal rigidity, possibly after one or more rectal bleeding episodes; sudden fall in temperature or blood pressure; and rising pulse rate (indicating shock).
During acute infection, plan care and activities to allow the patient as much rest as possible. Raise the side rails and use other safety measures, because the patient may become delirious. Assign him a room close to the nurses’ station so he can be checked often. Use a room
deodorizer (preferably electric) to minimize odor from diarrhea and to provide a comfortable atmosphere for rest.
Accurately record intake and output. Maintain adequate I.V. hydration. When the patient can tolerate oral feedings, encourage highcalorie fluids such as milkshakes. Watch for constipation.
Provide good skin and mouth care. Turn the patient frequently, and perform mild passive exercises, as indicated. Apply mild heat to the abdomen to relieve cramps.
Don’t administer antipyretics. These mask fever and lead to possible hypothermia. Instead, to promote heat loss through the skin without causing shivering (which keeps fever high by vasoconstriction), apply tepid, wet towels (don’t use alcohol or ice) to the patient’s groin and axillae. To promote heat loss by vasodilation of peripheral blood vessels, use additional wet towels on the arms and legs, wiping with long, vigorous strokes.
After draining the abscesses of a joint, provide heat, elevation, and passive rangeof-motion exercises to decrease swelling and maintain mobility.
If the patient has positive stool cultures on discharge, tell him to be sure to wash his hands after using the bathroom and to avoid preparing uncooked foods, such as salads, for family members. He also shouldn’t work as a food handler until cultures are negative. (See Preventing recurrence of salmonellosis, page 835.)
Report all cases to public health authorities.
Electrolyte imbalances
Metabolic acidosis
Shock
Conjunctivitis
Urethritis
Arthritis
Rectal prolapse
Bacterial infection
demonstrate Shigella. Severe infection increases hemagglutinating antibodies. Sigmoidoscopy or proctoscopy may reveal typical superficial ulcerations.
To prevent dehydration, administer I.V. fluids as ordered. Measure intake and output (including stools) carefully.
Correct identification of Shigella requires examination and culture of fresh stool specimens. Therefore, hand carry specimens directly to the laboratory. Because shigellosis is suspected, include this information on the laboratory slip.
Use a disposable hot-water bottle to relieve abdominal discomfort, and schedule care to conserve patient strength.
During shigellosis outbreaks, obtain stool specimens from all potentially infected staff, and instruct those infected to remain away from work until two stool specimens are negative.
Basic food safety precautions and disinfection of drinking water prevents shigellosis from food and water contamination.
Report cases to the local health authorities.
Bacteremia
Severe dehydration
Life-threatening electrolyte disturbances
Acidosis
Shock
Hemolytic-uremic syndrome
Keep accurate intake and output records. Measure stool volume and note the presence of blood or pus. Replace fluids and electrolytes as needed, monitoring for decreased serum sodium and chloride levels and signs of gramnegative shock. Watch for signs of dehydration, such as poor skin turgor and dry mouth.
For infants, use contact precautions, give nothing by mouth, administer antibiotics as ordered, and maintain body warmth.
Prevent direct patient contact during epidemics. Report cases to local public health authorities. E. coli 0157:H7 is a reportable disease.
Use proper hand-washing technique. Teach health care personnel, patients, and their families to do the same.
Follow standard precautions. Provide the patient with a private room, wear protective clothing as necessary, such as when handling feces or soiled linens, and perform scrupulous hand washing before entering and after leaving the patient’s room.
Advise travelers to foreign countries to avoid unbottled water and uncooked fruits and vegetables.
superinfections of various parts of the body, and a rare disease called melioidosis. (See Melioidosis.) This bacillus is also associated with bacteremia, endocarditis, and osteomyelitis in drug addicts. In local Pseudomonas infections, treatment is usually successful and complications rare; however, in patients with any type of lowered immunologic resistance—premature neonates; elderly patients; patients with debilitating disease, burns, or wounds; or patients receiving chemotherapy or radiation therapy— septicemic Pseudomonas infections are serious and commonly fatal.
Septic shock
Severe mucopurulent pneumonia
Systemic inflammatory response syndrome
Multiple organ dysfunction
Death
with an antipseudomonal penicillin, such as ticarcillin or piperacillin. An alternative combination is amikacin and a similar penicillin or imipenem and cilastatin. Such combination therapy is necessary because Pseudomonas quickly becomes resistant to ticarcillin alone.
Use strict sterile technique when caring for I.V. lines, catheters, and other tubes.
Use suction catheters only once.
Properly dispose of suction bottle contents.
Label and date solution bottles and change them frequently, according to policy.
Change water for fresh flowers in the patient’s room daily.
Avoid using humidifiers in the patient’s room.
Observe and record the character of wound exudate and sputum.
Before administering antibiotics, ask the patient about a history of drug allergies, especially to penicillin. If combinations of piperacillin or ticarcillin and an aminoglycoside are ordered, schedule the doses 1 hour apart (ticarcillin may decrease the antibiotic effect of the aminoglycoside). Don’t give both antibiotics through the same administration set.
Monitor the patient’s renal function (output, blood urea nitrogen level, specific gravity, urinalysis, and creatinine level) during treatment with aminoglycosides. Obtain drug levels to ensure effectiveness.
Protect immunocompromised patients from exposure to this infection. Proper hand washing and sterile techniques prevent further spread. (See Preventing Pseudomonas infection.)
Hypoglycemia
Severe electrolyte depletion
Hypovolemic shock
Metabolic acidosis
Renal failure
Liver failure
Bowel ischemia
Bowel infarction
painless, profuse, watery diarrhea and effortless vomiting (without preceding nausea). As diarrhea worsens, the stools contain white flecks of mucus (rice-water stools). Because of massive fluid and electrolyte losses from diarrhea and vomiting (fluid loss in adults may reach 1 L/hour), cholera causes intense thirst, weakness, loss of skin turgor, wrinkled skin, sunken eyes, pinched facial expression, muscle cramps (especially in the extremities), cyanosis, oliguria, tachycardia, tachypnea, thready or absent peripheral pulses, falling blood pressure, fever, and inaudible, hypoactive bowel sounds.
Wear gloves when handling fecescontaminated articles and wash your hands after leaving the patient’s room.
Use contact precautions for diapered or incontinent persons for the duration of illness or to control institutional outbreaks.
Monitor output (including stool volume) and I.V. infusion accurately. To detect overhydration, carefully observe neck veins, take serial patient weights, and auscultate the lungs (fluid loss in cholera is massive, and improper replacement may cause potentially fatal renal insufficiency).
Protect the patient’s family by administering oral tetracycline or doxycycline, if ordered.
Advise anyone traveling to an endemic area to boil all drinking water and avoid uncooked vegetables and unpeeled fruits.
Report all cases to public health authorities.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree