23
Immunotherapeutic Agents
This chapter will be most useful after having a basic understanding of the material in Chapter 35, Immunosuppressants, Tolerogens, and Immunostimulants in Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 12th Edition. In addition to the material presented here, the 12th Edition contains:
• Figure 35-3 Generation of monoclonal antibodies that illustrates the method used to generate monoclonal antibodies
• The molecular structures of immunotherapeutic drugs
• A Case Study: Immunotherapy for Multiple Sclerosis that includes Table 35-3 Pharmacotherapy of Multiple Sclerosis listing specific pharmacotherapies for treating multiple sclerosis (MS)
LEARNING OBJECTIVES
 Understand the mechanisms of action of drugs used to suppress the immune response in organ transplantation and autoimmune diseases.
Understand the mechanisms of action of drugs used to suppress the immune response in organ transplantation and autoimmune diseases.
 Understand the mechanisms of action of drugs used to stimulate the immune system.
Understand the mechanisms of action of drugs used to stimulate the immune system.
 Know the untoward effects of immunotherapeutic drugs.
Know the untoward effects of immunotherapeutic drugs.
 Know the drugs used at each step in organ transplantation.
Know the drugs used at each step in organ transplantation.
 Know the drugs used in treating different autoimmune disorders.
Know the drugs used in treating different autoimmune disorders.
 Know which immunotherapeutic drugs are used in combination with other drugs.
Know which immunotherapeutic drugs are used in combination with other drugs.
DRUGS INCLUDED IN THIS CHAPTER
• Abatacept (ORENCIA)
• Adalimumab (HUMIRA)
• Aldesleukin (PROLEUKIN)
• Alefacept (AMEVIVE)
• Alemtuzumab (CAMPATH)
• Anakinra (KINERET)
• Azathioprine (IMURAN, others)
• Bacillus Calmette–Guerin (BCG; TICE BCG, THERACYS)
• Basiliximab (SIMULECT)
• Belatacept (NULOJIX)
• Canakinumab (ILARIS)
• Cyclosporine (NEORAL, SANDIMMUNE, GENGRAF, others)
• Daclizumab (ZENAPAX)
• Efalizumab (RAPTIVA)
• Etanercept (ENBREL)
• Everolimus
• Glatiramer acetate (GA; COPAXONE)
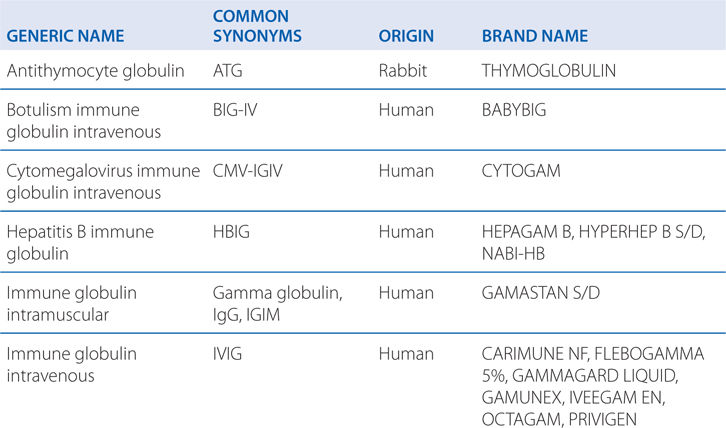
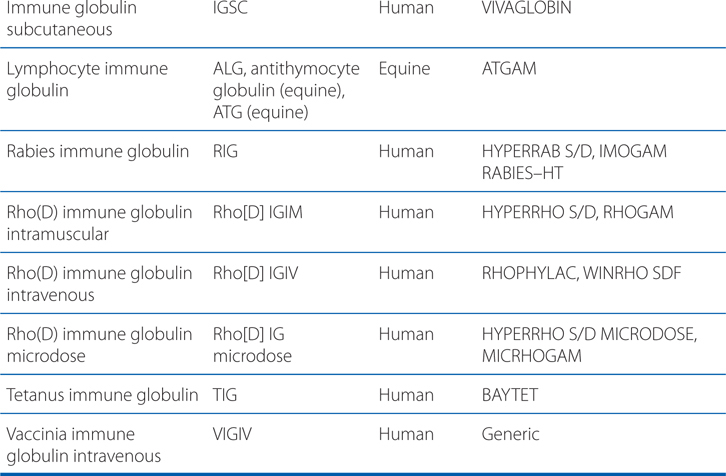
• Immune globulin preparations such as ATG (see Table 23-1)
TABLE 23-1 Selected Immune Globulin Preparations
• Infliximab (REMICADE)
• Interferon-α-2b (IFN-α-2b; INTRON A)
• Interferon-β-1a (IFN-β-1a; AVONEX, REBIF)
• Interferon-β-1b (IFN-β-1b; BETASERON)
• Interferon-γ-1b (IFN-γ-1b; ACTIMMUNE)
• Lenalidomide (REVLIMID)
• Mitoxantrone (NOVANTRONE, others)
• MPA (MYFORTIC)
• Muromonab-CD3 (OKT3, ORTHOCLONE OKT3)
• Mycophenolate mofetil (MMF, CELL-CEPT)
• Natalizumab (TYSABRI)
• Rilonacept (IL-1 TRAP)
• Sirolimus (rapamycin; RAPAMUNE)
• Some cancer chemotherapeutic agents (see Chapter 45)
• Tacrolimus (PROGRAF, FK506)
• Tacrolimus (PROGRAF, PROTOPIC, others)
• Thalidomide (THALOMID)
MECHANISMS OF ACTION OF DRUGS USED AS IMMUNOSUPPRESSANTS
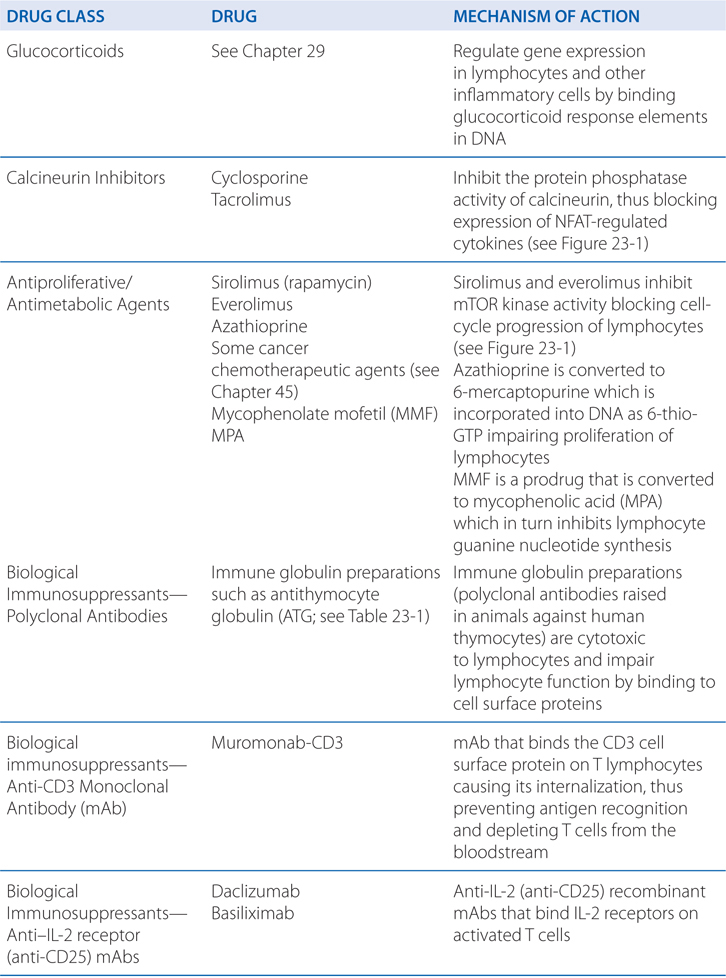
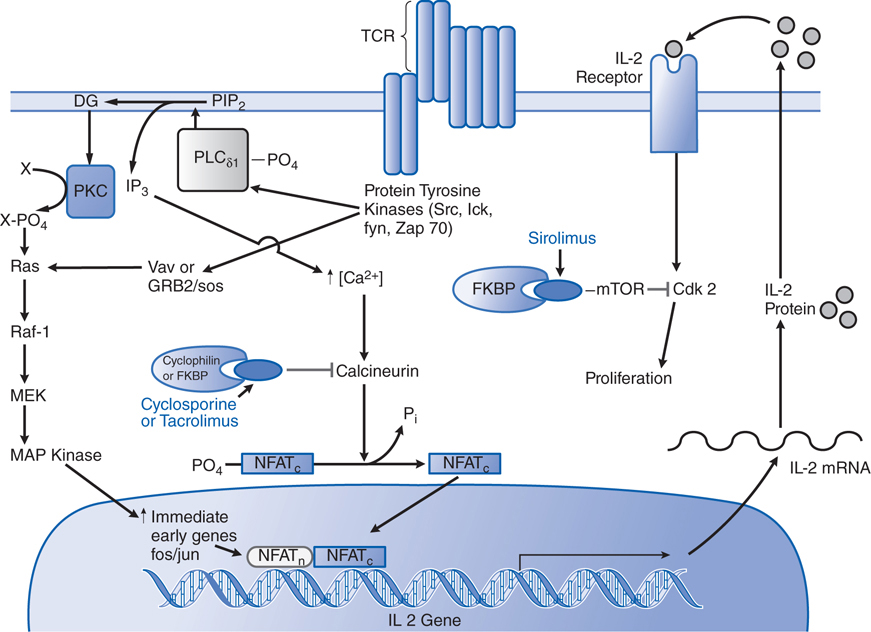
FIGURE 23-1 Mechanisms of action of cyclosporine, tacrolimus, and sirolimus on T lymphocytes. Both cyclosporine and tacrolimus bind to immunophilins (cyclophilin and FK506-binding protein [FKBP], respectively), forming a complex that binds the phosphatase calcineurin and inhibits the calcineurin-catalyzed dephosphorylation essential to permit movement of the nuclear factor of activated T cells (NFAT) into the nucleus. NFAT is required for transcription of interleukin-2 (IL-2) and other growth- and differentiation-associated cytokines (lymphokines). Sirolimus (rapamycin) works at a later stage in T-cell activation, downstream of the IL-2 receptor. Sirolimus also binds FKBP, but the FKBP-sirolimus complex binds to and inhibits the mammalian target of rapamycin (mTOR), a kinase involved in cell-cycle progression (proliferation). TCR, T-cell receptor. (Reproduced with permission from Pattison JM et al. Mechanisms of allograft rejection. In Neilson EG, Couser WG, eds. Immunologic Renal Diseases. Philadelphia, PA: Lippincott-Raven; 1997. http://lww.com.)
THE IMMUNE RESPONSE
• The immune system is composed of 2 complementary mechanisms, described as innate and adaptive immunity, capable of discriminating self from nonself (microbes and tumors).
• Characterisitics of innate (natural) immunity:
▶ Does not require priming
▶ Broadly reactive
▶ Relatively low affinity
▶ The most active component of the immune system early on in an immune response
▶ Major effectors include complement, granulocytes, monocytes/macrophages, natural killer cells, mast cells, and basophils
• Characterisitics of adaptive (learned) immunity:
▶ Antigen-specific
▶ Depends on antigen priming
▶ Can be very high affinity
▶ Becomes the more dominant component of the immune response over time
▶ Important in normal immune response to infection and tumors
▶ Mediates transplant (allograft) rejection and autoimmunity
▶ Major effectors include B and T lymphocytes
▶ B lymphocytes make antibodies (immunoglobulins)
▶ T lymphocytes function as helper, cytolytic, and regulatory (suppressor) cells
▶ Once activated by specific antigen recognition via cell surface receptors, B and T lymphocytes differentiate and divide, leading to release of soluble mediators (cytokines and lymphokines)
• Immunosuppressants are used to dampen the immune response in organ transplant and autoimmune disease.
• Tolerogens are used to induce a state of antigen-specific nonresponsiveness in organ transplantation and autoimmune diseases while maintaining immune functions that protect against opportunistic infections and secondary tumors.
• Immunostimulants are used to augment the immune system in some patients with infections, cancers, and immunodeficiency.
• Carefully prepare patient and select best available match for organ donor.
• Use a multitiered approach to immunosuppressive drug therapy with coadministration of several agents, each directed at a different molecular target in the allograft response.
• To gain early engraftment or to treat established rejection, use greater immunosuppression than used to maintain long-term immunosuppression.
• Carefully investigate each episode of transplant dysfunction to identify cause(s).
• An immunosuppressant drug should be reduced or withdrawn if its toxicity exceeds its benefit.
ADVERSE EFFECTS OF IMMUNOSUPPRESSANTS
• General suppression of the immune system increases the risk of opportunistic infections and secondary tumors.
• Calcineurin inhibitors are nephrotoxic.
• Glucocorticoids are diabetogenic and have many other adverse effects (see Chapter 29).
• Using drugs that have synergistic effects can reduce the doses needed for therapeutic efficacy and limit specific toxicities.
A 38-year-old male patient requires a kidney transplant because his kidneys have failed as the result of congenital renal disease. His biological daughter has agreed to donate one of her kidneys.
a. What is typically done prior to an organ transplant such as this?
Organ transplantation therapy is organized around 5 general principles (see Side Bar GENERAL PRINCIPLES OF ORGAN TRANSPLANT THERAPY). The first principle is careful patient preparation and selection of the best available ABO blood type–compatible human leukocyte antigen (HLA) match for organ donation. Because the organ donor in this case is the patient’s daughter, the match is likely to be excellent. By having a good match, the possibility of acute immune rejection is significantly reduced.
b. What drugs are used prophylactically to prevent organ transplant rejection?
Biological agents for induction therapy in the prophylaxis of rejection currently are used in ~70% of de novo transplant patients and have been propelled by several factors, including the introduction of the relatively safe anti–IL-2R antibodies and the emergence of antithymocyte globulin (ATG) as a safer and more effective alternative to lymphocyte immune globulin or muromonab-CD3. Induction therapy with biological agents is used to delay the use of the nephrotoxic calcineurin inhibitors or to intensify the initial immunosuppressive therapy in patients at high risk of rejection (ie, repeat transplants, broadly presensitized patients, African American patients, or pediatric patients).
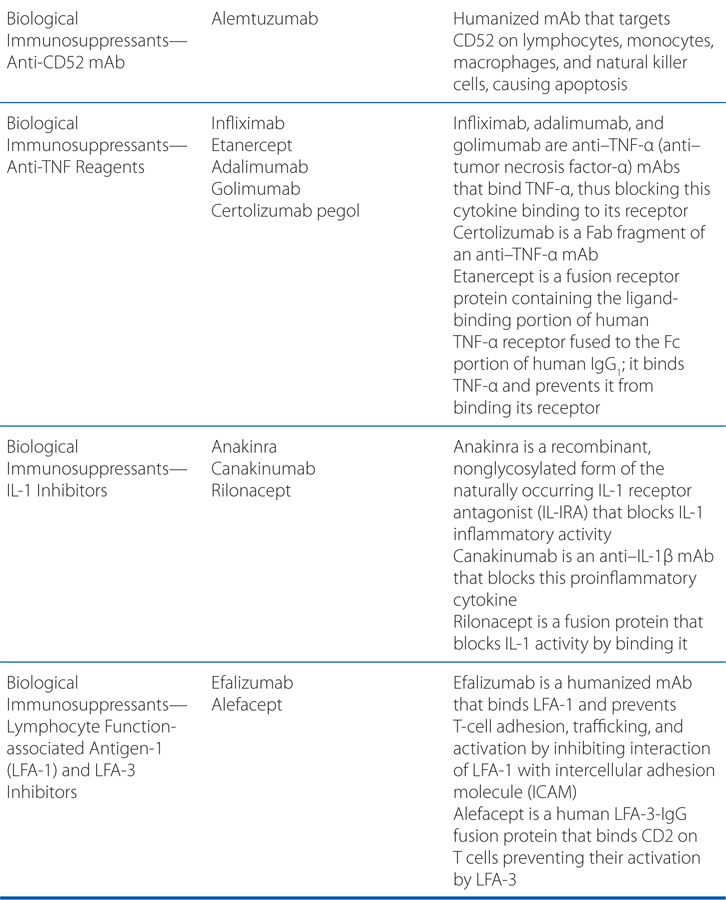
Biologicals for induction can be divided into 2 groups: the depleting agents and the immune modulators. The depleting agents consist of lymphocyte immune globulin, ATG, and muromonab-CD3 mAb (the latter also produces immune modulation); their efficacy derives from their ability to deplete the recipient’s CD3-positive cells at the time of transplant and antigen presentation. ATG is the most frequently used depleting agent. Lymphocyte immune globulin and OKT3 are rarely used because of poorer efficacy and acute side effects, respectively. Alemtuzumab, a humanized anti-CD52 monoclonal antibody that produces prolonged lymphocyte depletion, is increasingly used off-label as induction therapy in transplantation.
The second group of biological agents used for induction are the immune modulators, specifically the anti–IL-2R mAbs. These agents do not deplete T lymphocytes, with the possible exception of T regulatory cells, but rather block IL-2–mediated T-cell activation by binding to the α chain of IL-2R. These agents include anakinra, canakinumab, and rilonacept.
c. What is the approach to using immunosuppressants in organ transplants?
Immunosuppressive drugs are used to dampen the immune response in organ transplantation. A multitiered approach to immunosuppressive drug therapy is employed. Several agents, each of which is directed at a different molecular target within the allograft response (see MECHANISMS OF ACTION OF DRUGS USED AS IMMUNOSUPPRESSANTS), are used simultaneously. Synergistic effects permit use of the various agents at relatively low doses, thereby limiting specific toxicities while maximizing the immunosuppressive effect. Therapy typically involves a calcineurin inhibitor, glucocorticoids, and mycophenolate, each directed at a discrete site in T-cell activation. Glucocorticoids, azathioprine, cyclosporine, tacrolimus, mycophenolate, sirolimus, and various monoclonal and polyclonal antibodies all are approved for use in transplantation.
Greater immunosuppression is required to gain early engraftment and/or to treat established rejection than to maintain long-term immunosuppression. Maintenance immunosuppression consists of a calcineurin inhibitor (cyclosporine or tacrolimus), glucocorticoids, and an antimetabolite (azathioprine or mycophenolate). Mycophenolate has largely replaced azathioprine as part of the standard immunosuppressive regimen after transplant.
d. What are the risks of immunosuppressant drugs?
Immunosuppressant therapies require lifelong use and nonspecifically suppress the entire immune system, exposing patients to considerably higher risks of infection and cancer. The calcineurin inhibitors and glucocorticoids, in particular, are nephrotoxic and diabetogenic, respectively, thus restricting their usefulness in a variety of clinical settings. To minimize risks after engraftment is achieved, lower-dose maintenance drug protocols are employed. In addition, a drug should be reduced or withdrawn if its toxicity exceeds its benefit.
e. What might cause organ transplant dysfunction?
There can be many causes of transplant dysfunction, including rejection, drug toxicity, and infection. These various problems can and often do coexist. Organ-specific problems (eg, obstruction in the case of kidney transplants) also must be considered. A general principle of organ transplantation is to investigate each episode of transplant dysfunction to identify the cause(s) and adjust therapy.
A 62-year-old woman suffers from severe rheumatoid arthritis. Her pharmacotherapy includes several agents that are also commonly used for organ transplant pharmacotherapy.
a. What is the therapeutic rationale in using immunosuppressant drugs to treat this patient’s arthritis?
Rheumatoid arthritis is an autoimmune disease driven largely by activated T cells, giving rise to T cell–derived cytokines, such as IL-1 and TNF-α. Nonsteroidal anti-inflammatory drugs (NSAIDs) are widely used in patients with rheumatoid arthritis to relieve symptoms of pain and reduce inflammation (see Chapter 22), but they have minimal effect on disease progression and joint deformity. DMARDs (disease-modifying antirheumatic drugs), on the other hand, reduce the disease activity of rheumatoid arthritis and retard the progression of arthritic tissue destruction. These include a diverse group of small molecule nonbiologicals and biological agents (mainly antibodies or binding proteins), as summarized in Table 22-3, Disease-Modifying Antirheumatic Drugs. Many of the agents listed in Table 22-3 include the immunosuppressants covered in this chapter, including cyclosporine, azathioprine, TNF-α antagonists (infliximab, adalimumab, etanercept), IL-1 receptor antagonists (anakinra), and costimulatory blockers (abatacept).
Cyclosporine is used in severe cases of rheumatoid arthritis that have not responded to methotrexate. It can be combined with methotrexate, but the levels of both drugs must be monitored closely. Azathioprine is used in patients with severe rheumatoid arthritis. Lower initial doses of azathioprine are used for rheumatoid arthritis than used to prevent organ rejection. Biological DMARDs remain reserved for patients with persistent moderate or high disease activity and indicators of poor prognosis such as functional impairment, radiographic bony erosions, extra-articular disease, and rheumatoid factor positivity. Therapy is tailored to the individual patient, and the use of these agents must be weighed against their potentially serious adverse effects. Infliximab is approved in the United States for treating the symptoms of rheumatoid arthritis and is typically used in combination with methotrexate in patients who do not respond to methotrexate alone. Etanercept is approved for treatment of rheumatoid arthritis in patients who have not responded to other treatments and can be used in combination with methotrexate in patients who have not responded adequately to methotrexate alone.
INDUCTION OF IMMUNOLOGICAL TOLERANCE BY COSTIMULATORY BLOCKADE
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree