High-Yield Terms to Learn
Antigen-presenting cells (APCs) Dendritic and Langerhans cells, macrophages, and B lymphocytes involved in the processing of proteins into cell surface forms recognizable by lymphoid cells B cells Lymphoid cells derived from the bone marrow that mediate humoral immunity through the formation of antibodies Clusters of differentiation (CDs) Specific cell surface constituents identified by number (eg, CD4, CD8) Cytokines Polypeptide modulators of cellular functions, including interferons, interleukins, and growth-stimulating factors Immunophilins A family of cytoplasmic proteins that bind to the immunosuppressants cyclosporine, tacrolimus, and sirolimus and assist these drugs in inhibiting T- and B-cell function Major histocompatibility complex (MHC) Cell surface molecules that bind antigen fragments and, when bound to antigen fragments, are recognized by helper T cells. MHC class I molecules are expressed by all cells, whereas MHC class II molecules are expressed by antigen-presenting cells Monoclonal antibody (MAb) An antibody produced by a hybridoma clone that selectively binds to an antigen of biological or medical interest. T cells Lymphoid cells derived from the thymus that mediate cellular immunity and can modify humoral immunity. The main subclasses of T cells are CD4 (helper) cells and CD8 (cytotoxic) cells
Immune Mechanisms
Overview
Using the concerted actions of complement components, phagocytic cells, and natural killer (NK) cells, the innate immune system initiates the defense against pathogens and antigenic insult. If the innate response is inadequate, the adaptive immune response is mobilized. This culminates in the activation of T lymphocytes, the effectors of cell-mediated immunity, and the production of antibodies, by activated B lymphocytes, the effectors of humoral immunity. The subsets of lymphocytes that mediate different parts of the immune response can be identified by specific cell surface components or clusters of differentiation (CDs). For example, helper T (TH) cells bear the CD4 protein complex, whereas cytotoxic T lymphocytes express the CD8 protein complex.
Antigen Recognition and Processing
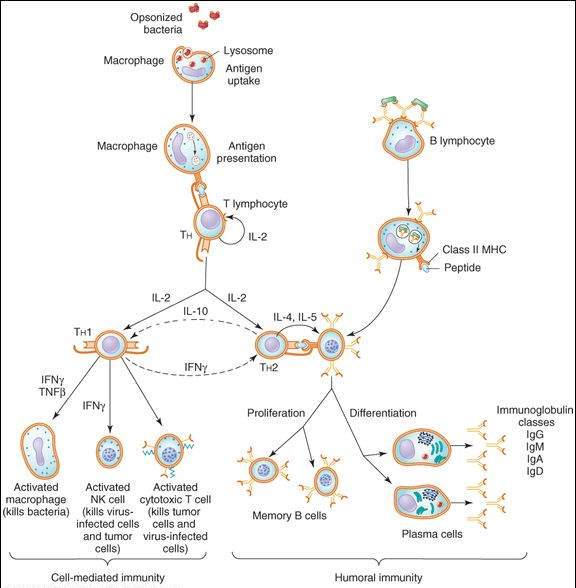
This critical inaugural step in the adaptive immune response involves antigen-presenting cells (APCs), which process antigens into small peptides that can be recognized by T-cell receptors (TCRs) on the surface of CD4 TH cells (Figure 55-1). The most important antigen-presenting cell surface molecules are the major histocompatibility complex (MHC) class I and II proteins. The activation of TH cells by the class II MHC-peptide complex requires the participation of costimulatory and adhesion molecules in addition to activation of T-cell receptors.
FIGURE 55-1
Scheme of cell-mediated and humoral immune responses. An immune response is initiated by internalization and processing of antigen by an antigen-presenting cell such as a macrophage. The class II MHC-peptide complex is recognized by the T-cell receptor (TCR) on T-helper (TH) lymphocytes, resulting in T-cell activation. Activated (TH) cells secrete cytokines such as IL-2, which cause proliferation and activation of TH1 and TH2 cells. TH1 cells produce IFN- and TNF-
and TNF-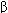 , which activate macrophages and NK cells. A humoral response is triggered when B lymphocytes bind antigen via their surface immunoglobulins. They are then induced by TH2-derived cytokines (eg, IL-4, IL-5) to proliferate and differentiate into memory cells and antibody-secreting plasma cells.
, which activate macrophages and NK cells. A humoral response is triggered when B lymphocytes bind antigen via their surface immunoglobulins. They are then induced by TH2-derived cytokines (eg, IL-4, IL-5) to proliferate and differentiate into memory cells and antibody-secreting plasma cells.
(Modified and reproduced, with permission, from Katzung BG, editor: Basic & Clinical Pharmacology, 11th ed. McGraw-Hill, 2009: Fig. 55-3.)
Cell-Mediated Immunity
Activated TH cells secrete interleukin-2 (IL-2), a cytokine that initiates proliferation and activation of 2 subsets of helper T cells, TH1 and TH2 (Figure 55-1). TH1 cells orchestrate cell-mediated immunity and delayed hypersensitivity reactions. They produce interferon (IFN)- , IL-2, and tumor necrosis factor (TNF)-
, IL-2, and tumor necrosis factor (TNF)-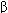 (also known as lymphotoxin). These cytokines activate macrophages, CD8 cytotoxic T lymphocytes (CTLs), and NK cells. Activated CTLs recognize processed peptides that are bound to class I MHC molecules on the surface of virus-infected or tumor cells. The CTLs induce target cell death via lytic enzyme and nitric oxide production and by stimulation of apoptosis pathways in the target cells. CTLs also play a role in autoimmune diseases by reacting against normal tissues, such as the synovium in rheumatoid arthritis and myelin in multiple sclerosis. NK cells kill both virus-infected and neoplastic cells.
(also known as lymphotoxin). These cytokines activate macrophages, CD8 cytotoxic T lymphocytes (CTLs), and NK cells. Activated CTLs recognize processed peptides that are bound to class I MHC molecules on the surface of virus-infected or tumor cells. The CTLs induce target cell death via lytic enzyme and nitric oxide production and by stimulation of apoptosis pathways in the target cells. CTLs also play a role in autoimmune diseases by reacting against normal tissues, such as the synovium in rheumatoid arthritis and myelin in multiple sclerosis. NK cells kill both virus-infected and neoplastic cells.
Humoral Immunity
The B lymphoid cells, which are capable of differentiating into antibody-forming cells, mediate humoral immunity. The humoral response is triggered when B lymphocytes bind antigen via their surface immunoglobulins. The antigens are internalized, processed into peptides, bound to MHC class II molecules, and presented on the B-cell surface. When T-cell receptors on TH2 cells are activated by the MHC II-peptide complex, they release interleukins (IL-4, IL-5, IL-6, IL-10, IL-13). These cytokines induce B-lymphocyte proliferation and differentiation into memory B cells and antibody-secreting plasma cells (Figure 55-1). Antibodies produced by plasma cells bind to antigens on the surface of pathogens and trigger the precipitation of viruses and the destruction of bacteria by phagocytic cells or lysis by the complement system.
The proliferation and differentiation of both B and T lymphocytes are under the control of a complex interplay between the cytokines (Table 55-1) and other endogenous molecules, including leukotrienes, and prostaglandins. For example, IL-10 and IFN- downregulate TH1 and TH2 responses, respectively (Figure 55-1).
downregulate TH1 and TH2 responses, respectively (Figure 55-1).
TABLE 55-1 Cytokines that modulate immune responses.
Cytokine Characteristic Properties Interferon- (IFN-
(IFN- ) Activates NK cells, antiviral, oncostatic Interferon-
) Activates NK cells, antiviral, oncostatic Interferon-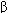 (IFN-
(IFN-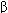 ) Activates NK cells, antiviral, oncostatic Interferon-
) Activates NK cells, antiviral, oncostatic Interferon- (IFN-
(IFN- ) Activates TH1, NK, cytotoxic T cells, and macrophages; antiviral, oncostatic Interleukin-2 (IL-2) T-cell proliferation, activation of TH1, NK, and LAK cells
) Activates TH1, NK, cytotoxic T cells, and macrophages; antiviral, oncostatic Interleukin-2 (IL-2) T-cell proliferation, activation of TH1, NK, and LAK cells
Interleukin-11 (IL-11) B-cell differentiation, megakaryocyte proliferation (see Chapter 33) Tumor necrosis factor- (TNF-
(TNF- ) Proinflammatory, macrophage activation, oncostatic Tumor necrosis factor-
) Proinflammatory, macrophage activation, oncostatic Tumor necrosis factor-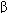 (TNF-
(TNF-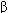 ) Proinflammatory, chemotactic, oncostatic Granulocyte colony-stimulating factor (G-CSF) Granulocyte production (see Chapter 33) Granulocyte-macrophage colony-stimulating factor (GM-CSF) Granulocyte, monocyte, eosinophil production (see Chapter 33)
) Proinflammatory, chemotactic, oncostatic Granulocyte colony-stimulating factor (G-CSF) Granulocyte production (see Chapter 33) Granulocyte-macrophage colony-stimulating factor (GM-CSF) Granulocyte, monocyte, eosinophil production (see Chapter 33)
Modified and reproduced, with permission, from Katzung BG, editor: Basic & Clinical Pharmacology, 11th ed. McGraw-Hill, 2009.
Abnormal Immune Responses
Abnormal immune responses include hypersensitivity, autoimmunity, and immunodeficiency states. Immediate hypersensitivity is usually antibody-mediated and includes anaphylaxis and hemolytic disease of the newborn. Delayed hypersensitivity, associated with extensive tissue damage, is cell-mediated. Autoimmunity arises from self-reactive lymphocytes that react to one’s own molecules, or self antigens. Examples of autoimmune diseases that are amenable to drug treatment include rheumatoid arthritis and systemic lupus erythematosus. Immunodeficiency states can be genetically acquired (eg, DiGeorge syndrome) or can result from extrinsic factors (eg, HIV infection).
Immunosuppressive Agents
The primary immunosuppressive agents are a diverse group of drugs that range from the corticosteroid hormonal drugs (discussed also in Chapter 39) to antimetabolite anticancer drugs (discussed also in Chapter 54) to drugs that more selectively target cells of the immune system.
Corticosteroids
Mechanism of Action
Glucocorticoids act at multiple cellular sites to cause broad effects on inflammatory and immune processes (see Chapter 39). At the biochemical level, their actions on gene expression decrease the synthesis of prostaglandins, leukotrienes, cytokines, and other signaling molecules that participate in immune responses (eg, platelet activating factor). At the cellular level, the glucocorticoids inhibit the proliferation of T lymphocytes and are cytotoxic to certain subsets of T cells. Although glucocorticoids impair cell-mediated immunity to the greatest extent, humoral immunity is also dampened and continuous therapy lowers IgG levels by increasing the catabolic rate of this class of immunoglobulins.
Clinical Use
Glucocorticoids are used alone or in combination with other agents in a wide variety of medical conditions that have an underlying undesirable immunologic reaction (see Chapter 39). They are also used to suppress immunologic reactions in patients who undergo organ transplantation and to treat hematologic cancers (see Chapter 54).
Toxicity
Predictable adverse effects include adrenal suppression, growth inhibition, muscle wasting, osteoporosis, salt retention, glucose intolerance, and behavioral changes (see Chapter 39).
Immunophilin Inhibitors
Mechanism of Action
These immunosuppressants interfere with T-cell function by binding to immunophilins, small cytoplasmic proteins that play critical roles in T-cell responses to T-cell receptor activation and to cytokines. Cyclosporine binds to cyclophilin and tacrolimus binds to FK-binding protein (FKBP). Both complexes inhibit calcineurin, a cytoplasmic phosphatase. Calcineurin regulates the ability of the nuclear factor of activated T cells (NFAT) to translocate to the nucleus and increase the production of key cytokines such as IL-2, IL-3, and IFN- . Cyclophilin and tacrolimus both prevent the increased production of cytokines that normally occurs in response to T-cell receptor activation. Sirolimus also binds to FKBP. However, this proliferation signal inhibitor interferes with the response of T cells to cytokines without affecting cytokine production. Sirolimus appears to also inhibit B-cell proliferation and antibody production.
. Cyclophilin and tacrolimus both prevent the increased production of cytokines that normally occurs in response to T-cell receptor activation. Sirolimus also binds to FKBP. However, this proliferation signal inhibitor interferes with the response of T cells to cytokines without affecting cytokine production. Sirolimus appears to also inhibit B-cell proliferation and antibody production.
Clinical Uses and Pharmacokinetics
Use of these immunosuppressants is a major factor in the success of solid organ transplantation. They are used in solid organ transplantation and to prevent and treat graft-versus-host (GVH) disease in recipients of allogeneic stem cell transplantation. These agents, particularly cyclosporine and tacrolimus, are also used in some autoimmune diseases, including rheumatoid arthritis, uveitis, psoriasis, asthma, and type 1 diabetes. Sirolimus-eluting stents are used to prevent restenosis after coronary angioplasty.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree