Immunomodulating Agent-associated Lymphoproliferative Disorders
Tariq Muzzafar, MBBS
Key Facts
Etiology/Pathogenesis
Risk factors for IA-LPDs
Type of immunosuppressive drug
Duration of drug therapy
Underlying disease type and disease activity
Patient genetic background
EBV plays role in subset of cases
Clinical Issues
Presentation and therapy similar to corresponding LPDs in immunocompetent patients
Methotrexate-associated LPD
Partial regression after drug withdrawal in subset of cases, especially if EBV(+)
TNF-α inhibitors
Regression after drug withdrawal is uncommon
HSTCL in Crohn disease
Fatal in most patients
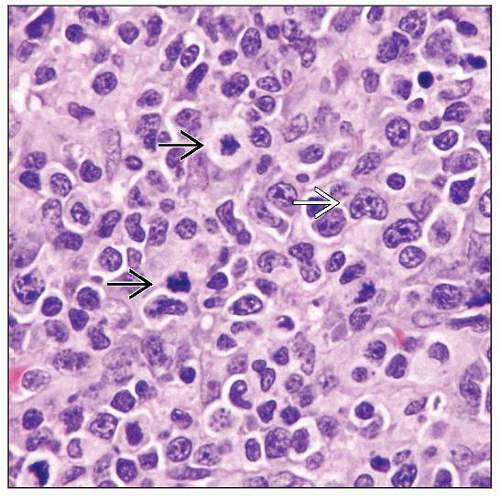
Microscopic Pathology
Many lymphoma types described in patients on immunomodulator therapy
Analogous to other immunodeficiency states; most common are
Diffuse large B-cell lymphoma
Classical Hodgkin lymphoma; Hodgkin-like LPD
Polymorphic/lymphoplasmacytic LPD
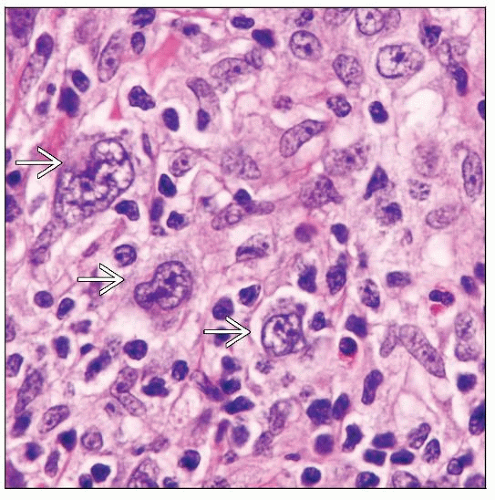
Hepatosplenic T-cell lymphoma
Ancillary Tests
Immunophenotype similar to corresponding LPDs in immunocompetent patients
Diagnostic Checklist
Knowledge of drug therapy essential for diagnosis
TERMINOLOGY
Abbreviations
Immunomodulating agent-associated lymphoproliferative disorders (IA-LPD)
Definitions
LPDs in patients treated with immunosuppressive drugs, usually for autoimmune diseases
LPDs arising in setting of transplantation are excluded
ETIOLOGY/PATHOGENESIS
Risk Factors for IA-LPD
Type of immunosuppressive drug
Methotrexate (MTX), tumor necrosis factor (TNF)-α antagonists, etc.
Duration of drug therapy
Underlying disease type and disease activity
Rheumatoid arthritis (RA) appears to have highest risk
Patient genetic background
Difficult to tease out relative contributions of these factors
Risk factors point to potential pathogenesis
Immunosenescence and Lymphomagenesis in RA
RA patients with lymphoma have mean age of 70 years (range 32-91)
Increased age is correlated with immunosenescence
B-cell immune dysregulation in RA could drive B cell expansion
B-cell autoimmune activity increased due to
Rheumatoid factor, anti-cyclic citrullinated peptide antibodies, and free light chains
Systemic inflammation
Elevated erythrocyte sedimentation rate, C-reactive protein
Elevated B-cell survival factors: B-cell activating factor (BAFF) and APRIL (a proliferation-inducing ligand)
Increased B cells infected by Epstein-Barr virus (EBV) in circulation
T-cell immune dysregulation in RA could lead to loss of tolerance
T cells have marked contraction in diversity and premature telomere shortening
Leads to permissive conditions for EBV(+) B-cell proliferation
Epstein-Barr Virus and Lymphomagenesis in Autoimmune Disease
Virus is often present in lymphomas arising in patients with immune dysregulation
Virus transforms primary B cells in vitro
EBV(+) B-cell proliferation could be due to
Immunosenescence in autoimmune disease
MTX activated lytic EBV infection in host cells
However, EBV can account for only fraction of increased risk
MTX withdrawal can lead to spontaneous regression of EBV(+) diffuse large B-cell lymphoma (DLBCL)
Lymphomas in RA Patients: General Considerations
RA is a multisystemic disease with increased risk of lymphoma
Risk correlates with cumulative inflammatory activity
Risk may be reduced with aggressive treatment by decreasing cumulative inflammation
DLBCL is most common type of lymphoma
Risk of DLBCL increased 100x from 1st to 3rd tertile of cumulative inflammation
Long duration of RA before diagnosis of lymphoma; mean: 20 years (range: 4-50 years)
RA Patients Treated with Methotrexate
Methotrexate is a potent immunosuppressive agent
Activates lytic EBV infection in host cells
Appears to cause IA-LPD in at least some patients in whom drug cessation leads to regression
Regression is more common in EBV(+) IA-LPDs
Complete or partial remission usually occurs within 4 weeks of cessation; remission sustained
However, no increase in risk of lymphoma attributable to MTX demonstrated in large, population-based studies
Risk associated with MTX therapy may appear falsely elevated due to selection bias
Patients on immunomodulating therapy are more likely to have active disease
Types of lymphomas described in patients treated with MTX
DLBCL (˜ 50% of cases)
Classical Hodgkin lymphoma (CHL) (20% of cases)
Polymorphic/lymphoplasmacytic LPD (15% of cases)
Follicular lymphoma (˜ 10% of cases)
Peripheral T-cell lymphoma (rare)
Risk of DLBCL correlates with prolonged duration of RA, therapy, and dose of MTX
Duration of RA: Median 96 months
Duration of MTX treatment: Median 56 months
Cumulative MTX dose: Median ˜ 900 mg
RA Patients Treated with Azathioprine
Risk of lymphoma increased
Lower risk than patients treated with MTX
RA Patients Treated with TNF-α Antagonists
Drugs include
Infliximab, Adalimumab, Etanercept
Current data indicate that treatment up to 4 years does not increase risk
DLBCL and CHL have been reported
Risk of lymphoma is difficult to estimate because
TNF-α antagonists are administered to RA patients with most severe disease
Underlying risk for lymphoma is very high in these patients
These drugs are often combined with MTX or used in patients who previously received MTX
Polymorphous LPDs that do not meet criteria for lymphoma can regress with drug cessation
RA Patients Treated with Other Drugs
Risk of lymphoma does not appear to be increased in patients treated with intramuscular gold or sulfasalazine
Risk decreased with oral steroids (odds ratio of 0.6); and intraarticular steroids
Risk of lymphoma not yet clear for rituximab, Abatacept, Anakinra
Crohn Disease and Lymphoma
Risk of LPD in inflammatory bowel disease increased
˜ 2x increase independent of therapy
DLBCL (most common); T-cell lymphomas, CHL reported
Risk increased further by therapy with azathioprine and 6-mercaptopurine (6-MP)
DLBCL, MALT lymphoma, CHL, and plasmacytoma
˜ 40% of these are EBV(+)
Risk with infliximab
Incidence of LPDs in patients receiving infliximab for Crohn disease is 0.2-1.4%
Small subset of cases have regressed following drug cessation
T-cell lymphomas have been reported
HSTCL (n=8), Sézary syndrome (n=2)
Systemic anaplastic large cell lymphoma (n=1), cutaneous CD30(+) T-cell lymphoma (n=1)
Infliximab may predispose to or cause lymphomagenesis due to
Impaired T-cell apoptosis leading to decreased activated T cells in peripheral blood
Impaired T-cell immune surveillance
Crohn disease and HSTCL
100% of patients were treated with azathioprine or 6-MP in past
4-year gap between thiopurine therapy and development of HSTCL
˜ 80% of patients had prior treatment with infliximab
Interval between 1st dose and development of HSTCL: Median 33 months
No reported cases of HSTCL in patients treated only with TNF-α inhibitor
Causal role of infliximab remains unproven
CLINICAL ISSUES
Epidemiology
Incidence
Not well characterized
Overall risk of LPDs increased 2x in RA
Severe disease activity associated with higher risk
Concurrent treatment with ≥ 2 immunomodulator agents confounds risk assessment for specific drug
Age
DLBCL
Median: 62 years
HSTCL in Crohn disease
Median at diagnosis: 22 years (range: 12-40 years)
Gender
Sex ratio related to underlying disease in most instances
HSTCL in Crohn disease: ˜ 90% of patients are male
Site
Methotrexate-associated LPD
˜ 50% are extranodal
Gastrointestinal (GI) tract, liver, spleen, lung, kidney
Skin, soft tissue, thyroid gland, bone marrow
Classical Hodgkin lymphoma
Usually involves lymph nodes
HSTCL
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree