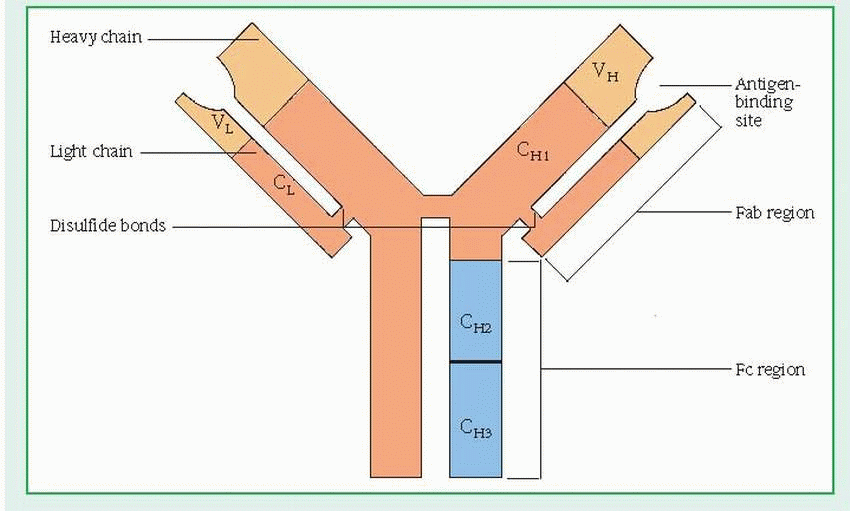
(H) and two light (L) chains. (See Structure of the immunoglobulin molecule, page 392.) Each chain has a variable (V) region and one or more constant (C) regions, which are coded by separate genes. The V regions of both L and H chains participate in the binding of antigens. The C regions of the H chain provide a binding site for crystallizable fragment (Fc) receptors on cells and govern other mechanisms.
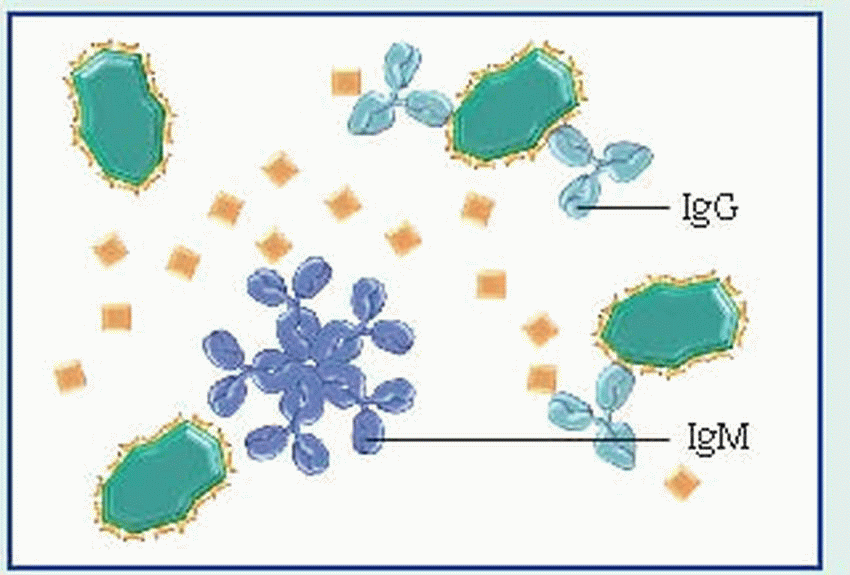
IgG, the smallest immunoglobulin, appears in all body fluids because of its ability to move across membranes as a single structural unit (a monomer). It constitutes 75% of total serum immunoglobulins and is the major antibacterial and antiviral antibody.
IgM, the largest immunoglobulin, appears as a pentamer (five monomers joined by a J-chain). Unlike IgG—which is produced mainly in the secondary, or recall, response—IgM dominates in the primary, or initial, immune response. However, like IgG, IgM is involved in classic antibody reactions, including precipitation, agglutination, neutralization, and complement fixation. Because of its size, IgM can’t readily cross membrane barriers and is usually present only in the vascular system. IgM constitutes 5% of total serum immunoglobulins.
IgA exists in serum primarily as a monomer; in secretory form, IgA exists almost exclusively as a dimer (two monomer molecules joined by a J-chain and a secretory component chain). As a secretory immunoglobulin, IgA defends external body surfaces and is present in colostrum, saliva, tears, nasal fluids, and respiratory, GI, and genitourinary secretions. This antibody is considered important in preventing antigenic agents from attaching to epithelial surfaces. IgA makes up 20% of total serum immunoglobulins.
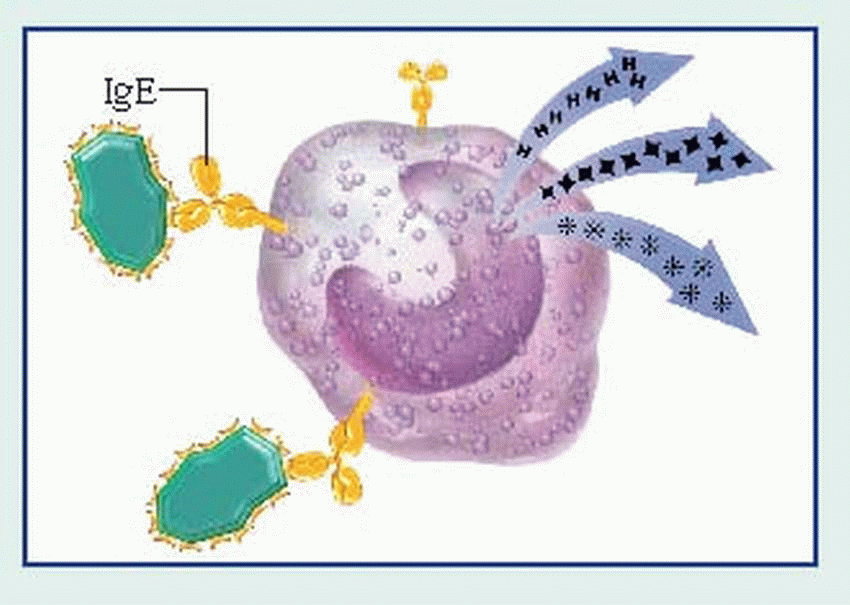
IgE, present in trace amounts in serum, is involved in the release of vasoactive amines stored in basophils and tissue mast cell granules. When released, these bioamines cause the allergic effects characteristic of this type of hypersensitivity (erythema, itching, smoothmuscle contraction, secretions, and swelling).
IgD, present as a monomer in serum in minute amounts, is the predominant antibody found on the surface of B lymphocytes and serves mainly as an antigen receptor. It may function in controlling lymphocyte activation or suppression.
 |
antigen, then deposit it on their own surfaces in association with HLA gene. T lymphocytes become activated upon recognizing this complex. Macrophages also function in the inflammatory response by producing IL-1, which generates fever. Additionally, macrophages synthesize complement proteins and other mediators that produce phagocytic, microbicidal, and tumoricidal effects.
Colony-stimulating factors function primarily as hematopoietic growth factors, guiding the division and differentiation of bone marrow stem cells. They also influence the functioning of mature lymphocytes, monocytes, macrophages, and neutrophils.
Interferons act early to limit the spread of viral infections. They also inhibit tumor growth. Mainly, they determine how well tissue cells interact with cytotoxic cells and lymphocytes.
Interleukins are a large group of cytokines. (Those produced primarily by T lymphocytes are called lymphokines. Those produced by mononuclear phagocytes are called monokines.) They have a variety of effects, but most direct other cells to divide and differentiate.
Tumor necrosis factor is believed to play a major role in mediating inflammation and cytotoxic reactions (along with IL-1, IL-6, and IL-8).
Transforming growth factor demonstrates both inflammatory and anti-inflammatory effects. It’s believed to be partially responsible for tissue fibrosis associated with many diseases. It demonstrates immunosuppressive effects on T cells, B cells, and NK cells.
exaggerated, misdirected, or either absent or depressed, the result may be a hypersensitivity disorder, autoimmunity, or immunodeficiency, respectively. Some forms of immunodeficiency are iatrogenic. (See Iatrogenic immunodeficiency.)
Cytotoxic drugs. These drugs kill immunocompetent cells while they’re replicating. However, most cytotoxic drugs aren’t selective and thus interfere with all rapidly proliferating cells. As a result, they reduce the number of lymphocytes as well as phagocytes. Besides depleting their number, cytotoxic drugs interfere with lymphocyte synthesis and release of immunoglobulins and lymphokines.
Cyclophosphamide, a potent and commonly used immunosuppressant, initially depletes the number of B cells, suppressing humoral immunity. However, chronic therapy also depletes T cells, suppressing cell-mediated immunity as well. Cyclophosphamide may be used in systemic lupus erythematosus, Wegener’s granulomatosis, other systemic vasculitides, and in certain autoimmune disorders. Because it nonselectively destroys rapidly dividing cells, this drug can cause severe bone marrow suppression with neutropenia, anemia, and thrombocytopenia; gonadal suppression with sterility; alopecia; hemorrhagic cystitis; and nausea, vomiting, and stomatitis. It may also increase the risk of lymphoproliferative neoplasms.
Among other cytotoxic drugs used for immunosuppression are azathioprine (commonly used in kidney transplantation) and methotrexate (occasionally used in rheumatoid arthritis and other autoimmune disorders).
If the patient is receiving cytotoxic drugs, monitor his white blood cell (WBC) count. If it falls too low, the drug dosage may need to be adjusted. Also monitor urine output and watch for signs of cystitis, especially if the patient is taking cyclophosphamide. Ensure adequate fluid intake (2 qt [about 2 L] daily). Give mesna as ordered to help prevent hemorrhagic cystitis. Provide antiemetics to relieve nausea and vomiting as ordered. Give meticulous oral hygiene and report signs of stomatitis.
Teach the patient about the early signs and symptoms of infection. If his WBC count falls too low, granulocyte colony-stimulating factor may be used to boost the count. Make sure the male patient understands the risk of sterility; advise sperm banking if appropriate. Young women may take hormonal contraceptives to minimize ovarian dysfunction and to prevent pregnancy during administration of these potentially teratogenic drugs.
Corticosteroids. These adrenocortical hormones are used to treat immune-mediated disorders because of their potent anti-inflammatory and immunosuppressive effects. Corticosteroids stabilize the vascular membrane, blocking tissue infiltration by neutrophils and monocytes, thus inhibiting inflammation. They also “kidnap” T cells in the bone marrow, causing lymphopenia. Because these drugs aren’t cytotoxic, lymphocyte concentration can return to normal within 24 hours after they’re withdrawn. Corticosteroids also appear to inhibit immunoglobulin synthesis and to interfere with the binding of immunoglobulin to antigen or to cells with crystallizable fragment (Fc) receptors. These drugs have many other effects as well.
The most commonly used oral corticosteroid is prednisone. For long-term therapy, prednisone is best given early in the morning to minimize exogenous suppression of cortisol production and with food or milk to minimize gastric irritation. After the acute phase, it’s usually reduced to an alternate-day schedule and then gradually withdrawn to minimize potentially harmful adverse effects. Other corticosteroids used for immunosuppression include hydrocortisone, methylprednisolone, and dexamethasone.
Chronic corticosteroid therapy can cause numerous adverse effects, which are sometimes more harmful than the disease itself. Neurologic adverse effects include euphoria, insomnia, or psychosis; cardiovascular effects include hypertension and edema; and GI effects include gastric irritation, ulcers, and increased appetite with weight gain. Other possible effects are cataracts, hyperglycemia, glucose intolerance, muscle weakness, osteoporosis, delayed wound healing, and increased susceptibility to infection.
During corticosteroid therapy, monitor the patient’s blood pressure, weight, intake and output, and blood glucose. Instruct the patient to eat a well-balanced, low-salt diet or to follow the specially prescribed diet to prevent excessive weight gain. Remember that even though the patient is more susceptible to infection, he’ll show fewer or less dramatic signs of inflammation.
Cyclosporine. Cyclosporine selectively suppresses the proliferation and development of helper T cells, resulting in depressed cellmediated immunity. This drug is used primarily to prevent rejection of kidney, liver, and heart transplants but is also being investigated for use in several other disorders. Significant toxic effects of cyclosporine primarily involve the liver and kidney, so treatment with this drug requires regular evaluation of renal and hepatic function. Adjusting the dose or the duration of therapy helps minimize certain adverse effects.
Antilymphocyte serum or antithymocyte globulin (ATG). This anti-T cell antibody reduces T-cell number and function, thus suppressing cell-mediated immunity. It has been used effectively to prevent cell-mediated rejection of tissue grafts or transplants. Usually, ATG is administered immediately before the transplant and continued for some time afterward. Potential adverse effects include anaphylaxis and serum sickness. Occurring 1 to 2 weeks after injection of ATG, serum sickness is characterized by fever, malaise, rash, arthralgias and, occasionally, glomerulonephritis or vasculitis. It presumably results from the deposition of immune complexes throughout the body.
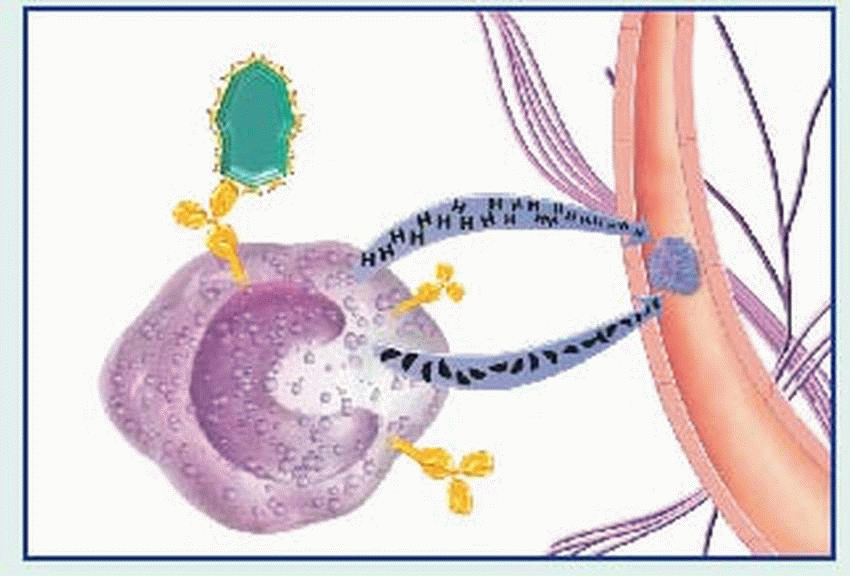
binds to the Fc receptors on the surface of mast cells. When these cells are re-exposed to the same antigen, the antigen binds with the surface IgE, cross-links the Fc receptors, and causes mast cell degranulation with release of various mediators. (Degranulation may also be triggered by complement-derived anaphylatoxins—C3a and C5a—or by certain drugs such as morphine.)
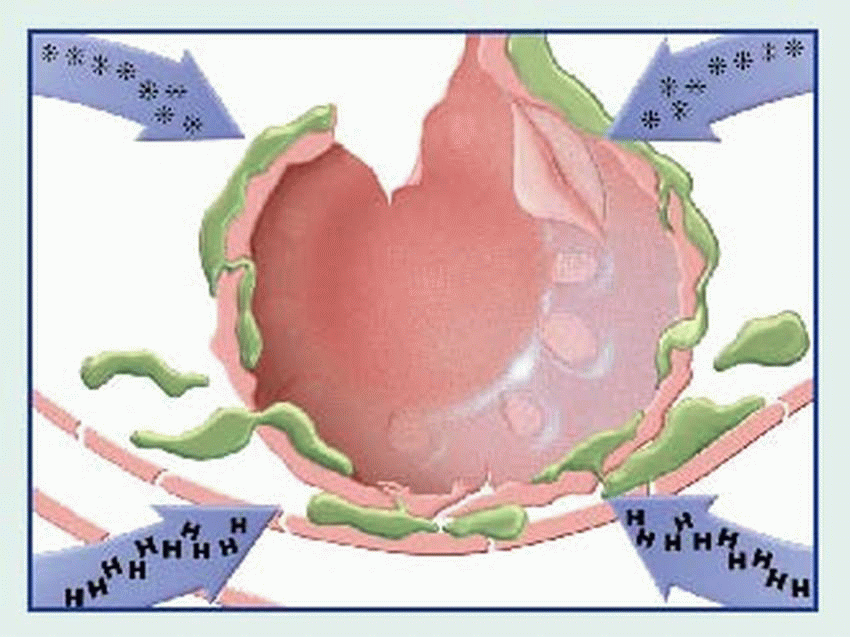
contraction, vasodilation, bronchospasm, edema, increased vascular permeability, mucus secretion, and cellular infiltration by eosinophils and neutrophils. Among classic associated signs and symptoms are hypotension, wheezing, swelling, urticaria, and rhinorrhea.
Type | Cause | Antibody or cell involved | Pathophysiology |
I—Immediate hypersensitivity (anaphylaxis, atopy) | Foreign protein (antigen) | Immunoglobulin (Ig)E | IgE attaches to the surface of the mast cell and specific antigen, and triggers release of intracellular granules from mast cells |
II—Cytotoxic hypersensitivity | Foreign protein (antigen) | IgG or IgM | IgG or IgM reacts with antigen, activates complement, and causes cytolysis or phagocytosis |
III—Immune complex disease | Foreign protein (antigen) Endogenous antigens | IgG, IgM, IgA | Antigen-antibody complexes precipitate in tissue, activate complement, and cause inflammatory reaction |
IV—Delayed cellmediated | Foreign protein, cell, or tissue | T lymphocytes | Sensitized T cells react with specific antigen to induce inflammatory process by direct cell action or by activity of lymphokines |
Clinical examples |
Extrinsic asthma, allergic rhinitis, anaphylaxis, reactions to stinging insects, some food and drug reactions |
Transfusion reaction, hemolytic drug reaction, Goodpasture’s syndrome, hemolytic disease of the neonate |
Rheumatoid arthritis, systemic lupus erythematosus, serum sickness to some drugs or viral hepatitis antigen, glomerulonephritis |
Contact dermatitis, graft-versus-host disease, granulomatous diseases |
Status asthmaticus
Respiratory failure
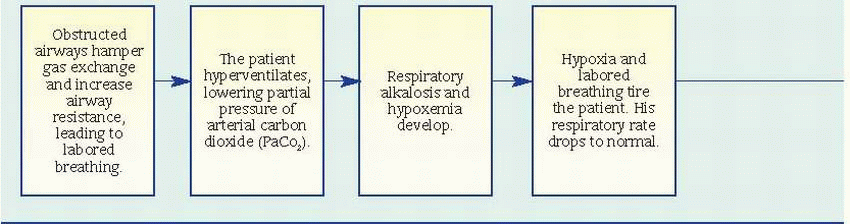
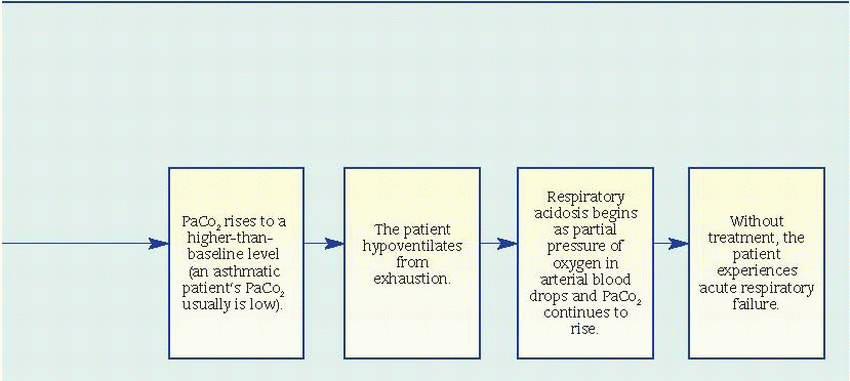
Pulmonary function studies reveal signs of airway obstruction (decreased peak expiratory flow rates and forced expiratory volume in 1 second), low-normal or decreased vital capacity, and increased total lung and residual capacity. However, pulmonary function studies may be normal between attacks.
Pulse oximetry may reveal decreased arterial oxygen saturation (SaO2).
Arterial blood gas (ABG) analysis provides the best indications of an attack’s severity. In acutely severe asthma, the partial pressure of arterial
oxygen is less than 60 mm Hg, the partial pressure of arterial carbon dioxide (PaCO2) is 40 mm Hg or more, and pH is usually decreased.
Complete blood count with differential reveals increased eosinophil count.
Chest X-rays may show hyperinflation with areas of focal atelectasis.
frequency, severity, and duration of symptoms
degree of airflow obstruction (spirometry measure) or peak expiratory flow (PEF)
frequency of nighttime symptoms and the degree to which the asthma interferes with daily activities
daytime symptoms no more than twice a week
nighttime symptoms no more than twice a month
lung function testing (either PEF or forced expiratory volume in 1 second) is 80% of predicted value or higher
PEF varies no more than 20%
daytime symptoms 3 to 6 days a week
nighttime symptoms 3 to 4 times a month
lung function testing is 80% of predicted value or higher
PEF varies between 20% and 30%
daily daytime symptoms
at least weekly nighttime symptoms
lung function testing is 60% to 80% of predicted value
PEF varies more than 30%
continual daytime symptoms
frequent nighttime symptoms
lung function testing is 60% of predicted value or lower
PEF varies more than 30%
fibrosis, tumors of the bronchi or mediastinum, and acute viral bronchitis; in adults, other causes include obstructive pulmonary disease, heart failure, and epiglottitis.
First, assess the severity of asthma.
Administer the prescribed treatments and assess the patient’s response.
Place the patient in high Fowler’s position. Encourage pursed-lip and diaphragmatic breathing. Help him to relax.
Administer prescribed humidified oxygen by nasal cannula at 2 L/minute to ease breathing and to increase SaO2. Later, adjust oxygen according to the patient’s vital signs and ABG levels.
Anticipate intubation and mechanical ventilation if the patient fails to maintain adequate oxygenation.
Monitor serum theophylline levels to ensure they’re in the therapeutic range. Observe your patient for signs and symptoms of theophylline toxicity (vomiting, diarrhea, and headache), as well as for signs of subtherapeutic dosage (respiratory distress and increased wheezing).
Observe the frequency and severity of your patient’s cough, and note whether it’s productive. Then auscultate his lungs, noting adventitious or absent breath sounds. If his cough isn’t productive and rhonchi are present, teach him effective coughing techniques. If the patient can tolerate postural drainage and chest percussion, perform these procedures to clear secretions. Suction an intubated patient as needed.
Treat dehydration with I.V. fluids until the patient can tolerate oral fluids, which will help loosen secretions.
If conservative treatment fails to improve the airway obstruction, anticipate bronchoscopy or bronchial lavage when a lobe or larger area collapses.
Monitor the patient’s respiratory status to detect baseline changes, to assess response to treatment, and to prevent or detect complications.
Auscultate the lungs frequently, noting the degree of wheezing and quality of air movement.
Review ABG levels, pulmonary function test results, and SaO2 readings.
If the patient is taking systemic corticosteroids, observe for complications, such as elevated blood glucose levels and friable skin and bruising.
Cushingoid effects resulting from long-term use of corticosteroids may be minimized by alternate-day dosage or use of prescribed inhaled corticosteroids.
If the patient is taking corticosteroids by inhaler, watch for signs of candidal infection in the mouth and pharynx. Using an extender device and rinsing the mouth afterward may prevent this.
Observe the patient’s anxiety level. Keep in mind that measures that reduce hypoxemia and breathlessness should help relieve anxiety.
Keep the room temperature comfortable and use an air conditioner or a fan in hot, humid weather.
Control exercise-induced asthma by instructing the patient to use a bronchodilator or cromolyn 30 minutes before exercise. Also instruct him to use pursed-lip breathing while exercising.
Teach the patient and his family to avoid known allergens and irritants.
Describe to the patient prescribed drugs, including their names, dosages, actions, adverse effects, and special instructions.
Teach the patient how to use a metered-dose inhaler. If he has difficulty using an inhaler, he may need an extender device to optimize drug delivery and lower the risk of candidal infection with orally inhaled corticosteroids.
If the patient has moderate to severe asthma, explain how to use a peak flow meter to measure the degree of airway obstruction. Tell him to keep a record of peak flow readings and to bring it to medical appointments. Explain the importance of calling the physician at once if the peak flow drops suddenly (may signal severe respiratory problems).
Tell the patient to notify the physician if he develops a fever above 100° F (37.8°), chest pain, shortness of breath without coughing or exercising, or uncontrollable coughing. An uncontrollable asthma attack requires immediate attention.
Teach the patient diaphragmatic and pursedlip breathing as well as effective coughing techniques.
Urge him to drink at least 3 qt (3 L) of fluids daily to help loosen secretions and maintain hydration.
Secondary sinus and middle ear infections
Nasal polyps
Nasal obstruction
In chronic vasomotor rhinitis, eye symptoms are absent, rhinorrhea is mucoid, and seasonal variation is absent.
In infectious rhinitis (the common cold), the nasal mucosa is beet red; nasal secretions contain polymorphonuclear, not eosinophilic, exudate; and signs and symptoms include fever and sore throat. This condition isn’t a recurrent seasonal phenomenon.
Before desensitization injections, assess the patient’s symptom status. Afterward, watch for adverse reactions, including anaphylaxis and severe localized erythema.
Monitor the patient’s compliance with prescribed drug treatment regimens. Also carefully note any changes in the control of his symptoms or any signs of drug misuse.
To reduce environmental exposure to airborne allergens, suggest that the patient sleep with the windows closed, avoid the countryside during pollination seasons, use air conditioning to filter allergens and minimize moisture and dust, and eliminate dust-collecting items, such as wool blankets, deep-pile carpets, and heavy drapes, from the home.
In severe and resistant cases, suggest that the patient consider drastic changes in lifestyle such as relocation to a pollen-free area either seasonally or year-round.
Be aware that some patients may develop chronic complications, including sinusitis and nasal polyps.
Scarring
Secondary infection
erythematous, weeping lesions. Eventually, the lesions become scaly and lichenified. Usually, they’re located in areas of flexion and extension, such as the neck, antecubital fossa, popliteal folds, and behind the ears. Patients with atopic dermatitis are prone to unusually severe viral infections, bacterial and fungal skin infections, ocular complications, and allergic contact dermatitis.
Monitor the patient’s compliance with drug therapy.
Teach the patient when and how to apply topical corticosteroids.
Emphasize the importance of regular personal hygiene using only water with little soap.
Be alert for signs and symptoms of secondary infection; teach the patient how to recognize them as well.
If the patient’s diet is modified to exclude food allergens, monitor his nutritional status.
Offer support to help the patient and his family cope with this chronic disorder.
Discourage use of laundry additives.
Dissuade the patient from scratching during urticaria to help prevent an infection.
patients with a history of asthma or other allergies, especially to bananas, avocados, tropical fruits, or chestnuts
patients with a history of multiple intraabdominal or genitourinary surgeries
patients who require frequent intermittent urinary catheterization
Respiratory obstruction
Systemic vascular collapse
The patient may describe dermatitis or mild respiratory distress when using latex gloves, inflating a balloon, or coming in contact with other latex products.
Urge the patient to wear an identification tag mentioning his latex allergy.
Teach the patient and family members how to use an epinephrine autoinjector.
Teach the patient to be aware of all latexcontaining products and to use vinyl or silicone products instead. Advise him that Mylar balloons don’t contain latex.
Make sure that items that aren’t available in a latex-free form, such as stethoscopes and blood pressure cuffs, are wrapped in cloth before they come in contact with a hypersensitive patient’s skin.
Place the patient in a private room or with another patient who requires a latex-free environment
When adding medication to an I.V. bag, inject the drug through the spike port, not the rubber latex port.
Adhesive bandages
Airways, nasogastric tubes
Blood pressure cuff, tubing, and bladder Catheters
Catheter leg straps
Dental devices
Elastic bandages
Electrode pads Fluid-circulating hypothermia blankets
Handheld resuscitation bag
Hemodialysis equipment
I.V. catheters
Latex or rubber gloves
Medication vials
Pads for crutches
Protective sheets
Reservoir breathing bags
Rubber airway and endotracheal tubes
Stethoscopes
Tape
Tourniquets
Adhesive tape
Balloons (excluding Mylar)
Cervical diaphragms
Condoms
Dishwashing gloves
Disposable diapers
Elastic stockings
Glue
Latex paint
Nipples and pacifiers
Racquet handles
Rubber bands
Tires
Have an emergency kit available to treat allergic reactions.
Take a detailed patient history, including penicillin allergy and other allergies. In an infant younger than age 3 months, check for penicillin allergy in the mother.
Never give penicillin to a patient who has had an allergic reaction to it.
Before giving penicillin to a patient with suspected penicillin allergy, refer the patient for skin and immunologic tests to confirm it.
Always tell a patient he’s going to receive penicillin before he takes the first dose.
Observe the patient carefully for
adverse effects for at least ½ hour after penicillin administration.
Be aware that penicillin derivatives also may elicit an allergic reaction.
Respiratory obstruction
Systemic vascular collapse
Death
of the upper respiratory tract results in hypopharyngeal and laryngeal obstruction (hoarseness, stridor, and dyspnea). This is an early sign of acute respiratory failure, which can be fatal. GI and genitourinary symptoms include severe stomach cramps, nausea, diarrhea, and urinary urgency and incontinence.
 |
 |
 |
 |
Teach the patient to avoid exposure to known allergens. A person allergic to certain foods or drugs must learn to avoid the offending food or drug in all its forms. A person allergic to insect stings should avoid open fields and wooded areas during the insect season. An anaphylaxis kit (epinephrine, antihistamine, and tourniquet) should also be carried whenever the patient with known severe allergic reactions goes outdoors In addition, every patient prone to anaphylaxis should wear a medical identification bracelet identifying his allergies.
If a patient must receive a drug to which he’s allergic, prevent a severe reaction by making sure he receives careful desensitization with gradually increasing doses of the antigen or advance administration of steroids. Of course, a person with a known allergic history should receive a drug with a high anaphylactic potential only after cautious pretesting for sensitivity. Closely monitor the patient during testing, and make sure you have resuscitative equipment and epinephrine ready. When any patient needs a drug with a high anaphylactic potential (particularly parenteral drugs), make sure he receives each dose under close medical observation
Closely monitor a patient undergoing diagnostic tests that use radiographic contrast media, such as excretory urography, cardiac catheterization, and angiography.
Infection from scratching
Laryngeal edema (upper respiratory tract involvement)
Severe abdominal colic (GI involvement)
drug history, including over-the-counter preparations (vitamins, aspirin, and antacids)
frequently ingested foods (strawberries, milk products, fish)
environmental influences (pets, carpet, clothing, soap, inhalants, cosmetics, hair dye, and insect bites and stings)
larger doses of specific antigens (determined by skin testing) are injected intradermally. After the triggering stimulus has been removed, urticaria usually subsides in a few days—except for drug reactions, which may persist as long as the drug is in the bloodstream.
Inform the patient receiving antihistamines of the possibility of drowsiness.
Suggest cool compresses to reduce swelling and pain. A cool bath may be used for large areas. Calamine lotion may be soothing as well.
Avoid hot baths, which can cause hives to return.
Advise the patient to avoid tight-fitting clothing.