Immature Teratoma
Esther Oliva, MD
Key Facts
Terminology
Malignant germ cell tumor composed of immature tissue derived from the 3 germ layers with variable admixture of mature tissues
Clinical Issues
3rd most common malignant germ cell tumor
Clinical history of mature cystic teratoma (multiple and ruptured) in same or contralateral ovary (rare)
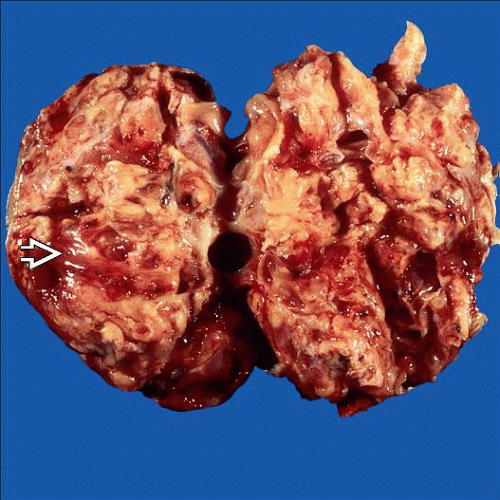
Macroscopic Features
Capsule rupture in ˜ 50%
Solid, or solid and cystic; fleshy, gray to pink
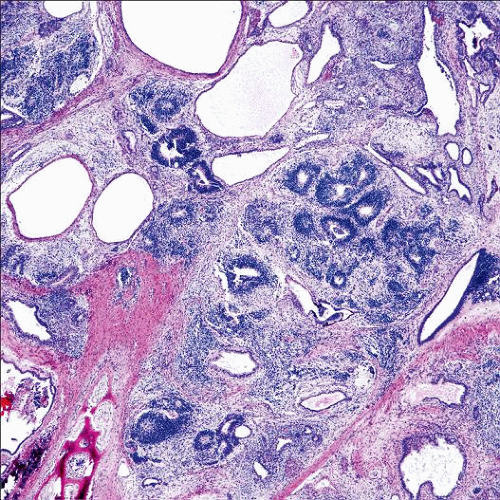
Microscopic Pathology
Variable admixture of mature and immature tissues derived from endoderm, mesoderm, and ectoderm
Immature neuroepithelium most common element (neuroepithelial rosettes, pseudo-rosettes, primitive tubules, or mitotically active glia)
Low-grade (grade I): 1 lower power field (4x) in any 1 slide
High-grade (grades II and III): > 1 low-power field in any 1 slide
Gliomatosis peritonei: 2-3 mm well-demarcated implants of mature (more commonly) or slightly immature glial tissue typically within peritoneum
Top Differential Diagnoses
Mature cystic teratoma with microscopic foci of neuroepithelium
Mature solid teratoma
Malignant neuroectodermal tumor
Malignant mixed mesodermal tumor
TERMINOLOGY
Abbreviations
Immature teratoma (IT)
Definitions
Malignant germ cell tumor composed of immature tissue derived from the 3 germ layers with variable admixture of mature tissues
ETIOLOGY/PATHOGENESIS
Neoplastic Transformation
From ovarian primordial germ cells
CLINICAL ISSUES
Epidemiology
Incidence
Rare
< 1% of all ovarian cancer in USA
20% of primitive germ cell tumors
3rd most common malignant germ cell tumor
2% of all ovarian teratomas
Age
Mostly first 2 decades
Presentation
Abdominal pain/swelling
Rapidly growing mass
Elevated AFP serum levels (typically < 1,000 ng/ml)
Elevated CA125 and CA19-9 serum levels common
Elevated HCG serum levels rare
Clinical history of mature cystic teratoma (multiple and ruptured) in same or contralateral ovary (rare)
Natural History
Growing teratoma syndrome
Usually during first 2 years after initial diagnosis and characterized by
Persistence or enlargement of pelvic peritoneal mass after chemotherapy
Low AFP levels
Absence of immature tissues within mass
Treatment
Unilateral salpingo-oophorectomy ± adjuvant chemotherapy (depending on grade and stage)
Prognosis
Very good after introduction of adjuvant chemotherapy
> 85% overall survival
Extraovarian spread in ˜ 1/3 at presentation
Gliomatosis peritonei; if present, higher risk of recurrence but similar overall survival to that of immature teratoma without gliomatosis
Lymph node involvement
Recurrences may occur (exceptional if confined to ovary and grade I)
MACROSCOPIC FEATURES