PART 19: Disorders Associated with Environmental Exposures
476e | Altitude Illness |
EPIDEMIOLOGY
Mountains cover one-fifth of the earth’s surface; 38 million people live permanently at altitudes ≥2400 m, and 100 million people travel to high-altitude locations each year. Skiers in the Alps or Aspen; religious pilgrims to Lhasa or Kailash; trekkers and climbers to Kilimanjaro, Aconcagua, or Everest; and military personnel deployed to high-altitude locations are all at risk of developing acute mountain sickness (AMS), high-altitude cerebral edema (HACE), high-altitude pulmonary edema (HAPE), and other altitude-related problems. AMS is the benign form of altitude illness, whereas HACE and HAPE are life-threatening. Altitude illness is likely to occur above 2500 m but has been documented even at 1500–2500 m. In the Mount Everest region of Nepal, ~50% of trekkers who walk to altitudes >4000 m over ≥5 days develop AMS, as do 84% of people who fly directly to 3860 m. The incidences of HACE and HAPE are much lower than that of AMS, with estimates in the range of 0.1–4%.
PHYSIOLOGY
Ascent to a high altitude subjects the body to a decrease in barometric pressure that results in a decreased partial pressure of oxygen in the inspired gas in the lungs. This change leads in turn to less pressure driving oxygen diffusion from the alveoli and throughout the oxygen cascade. A normal initial “struggle response” to such an ascent includes increased ventilation—the cornerstone of acclimation—mediated by the carotid bodies. Hyperventilation may cause respiratory alkalosis and dehydration. Alkalosis may depress the ventilatory drive during sleep, with consequent periodic breathing and hypoxemia. During early acclimation, renal suppression of carbonic anhydrase and excretion of dilute alkaline urine combat alkalosis and tend to bring the pH of the blood to normal. Other physiologic changes during normal acclimation include increased sympathetic tone; increased erythropoietin levels, leading to increased hemoglobin levels and red blood cell mass; increased tissue capillary density and mitochondrial numbers; and higher levels of 2,3-bisphosphoglycerate, enhancing oxygen utilization. Even with normal acclimation, however, ascent to a high altitude decreases maximal exercise capacity (by ~1% for every 100 m gained above 1500 m) and increases susceptibility to cold injury due to peripheral vasoconstriction. Finally, if the ascent is made faster than the body can adapt to the stress of hypobaric hypoxemia, altitude-related disease states can result.
GENETICS
![]() Hypoxia-inducible factor, which is important in high-altitude adaptation, controls transcriptional responses to hypoxia throughout the body and is involved in the release of vascular endothelial growth factor (VEGF) in the brain, erythropoiesis, and other pulmonary and cardiac functions at high altitudes. In particular, the gene EPAS1, which codes for transcriptional regulator hypoxia-inducible factor 2α, appears to play an important role in the adaptation of Tibetans living at high altitude, resulting in lower hemoglobin concentrations than are found in the Han Chinese. For acute altitude illness, a single gene variant is unlikely to be found, but the differences in the susceptibility of individuals and populations, familial clustering of cases, and a positive association of some genetic variants all clearly support a role for genetics. Approximately 58 candidate genes have been tested, and at least one variant from 17 of these genes is associated with altitude illness.
Hypoxia-inducible factor, which is important in high-altitude adaptation, controls transcriptional responses to hypoxia throughout the body and is involved in the release of vascular endothelial growth factor (VEGF) in the brain, erythropoiesis, and other pulmonary and cardiac functions at high altitudes. In particular, the gene EPAS1, which codes for transcriptional regulator hypoxia-inducible factor 2α, appears to play an important role in the adaptation of Tibetans living at high altitude, resulting in lower hemoglobin concentrations than are found in the Han Chinese. For acute altitude illness, a single gene variant is unlikely to be found, but the differences in the susceptibility of individuals and populations, familial clustering of cases, and a positive association of some genetic variants all clearly support a role for genetics. Approximately 58 candidate genes have been tested, and at least one variant from 17 of these genes is associated with altitude illness.
ACUTE MOUNTAIN SICKNESS AND HIGH-ALTITUDE CEREBRAL EDEMA
AMS is a neurologic syndrome characterized by nonspecific symptoms (headache, nausea, fatigue, and dizziness), with a paucity of physical findings, developing 6–12 h after ascent to a high altitude. AMS is a clinical diagnosis. For uniformity in research studies, the Lake Louise Scoring System, created at the 1991 International Hypoxia Symposium, is generally used. AMS must be distinguished from exhaustion, dehydration, hypothermia, alcoholic hangover, and hyponatremia. AMS and HACE are thought to represent opposite ends of a continuum of altitude-related neurologic disorders. HACE (but not AMS) is an encephalopathy whose hallmarks are ataxia and altered consciousness with diffuse cerebral involvement but generally without focal neurologic deficits. Progression to these signal manifestations can be rapid. Papilledema and, more commonly, retinal hemorrhages may also develop. In fact, retinal hemorrhages occur frequently at ≥5000 m, even in individuals without clinical symptoms of AMS or HACE. It is unclear whether retinal hemorrhage and cerebral hemorrhage at high altitude are caused by the same mechanism.
Risk Factors The most important risk factors for the development of altitude illness are the rate of ascent and a prior history of high-altitude illness. Exertion is a risk factor, but lack of physical fitness is not. An attractive but still speculative hypothesis proposes that AMS develops in people who have inadequate cerebrospinal capacity to buffer the brain swelling that occurs at high altitude. Children and adults seem to be equally affected, but people >50 years of age may be less likely to develop AMS than younger people. Aging appears to be associated with blunting of cardiac chronotropic function and an increase in ventilatory response leading to maintenance of arterial oxygen saturation in hypoxia. Most studies reveal no gender difference in AMS incidence. A recent study showed that, in women, adaptive responses to hypoxia with aging are blunted by menopause but can be maintained with endurance training. Sleep desaturation—a common phenomenon at high altitude—is associated with AMS. Debilitating fatigue consistent with severe AMS on descent from a summit is also an important risk factor for death in mountaineers. A recently published prospective study involving trekkers and climbers who ascended to altitudes between 4000 m and 8848 m showed that high oxygen desaturation and low ventilatory response to hypoxia during exercise are independent predictors of severe altitude illness. However, because there may be overlap between groups of susceptible and nonsusceptible individuals, accurate cutoff values are hard to define. Prediction is made more difficult because the pretest probabilities of HAPE and HACE are low. Neck irradiation or surgery damaging the carotid bodies, respiratory tract infections, and dehydration appear to be other potential risk factors for altitude illness.
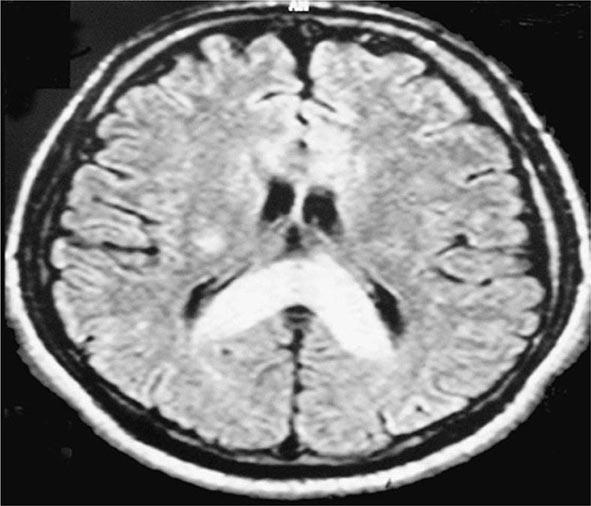
Pathophysiology The exact mechanisms causing AMS and HACE are unknown. Evidence points to a central nervous system process. MRI studies have suggested that vasogenic (interstitial) cerebral edema is a component of the pathophysiology of HACE. In the setting of high-altitude illness, the MRI findings shown in Fig. 476e-1 are confirmatory of HACE, with increased signal in the white matter and particularly in the splenium of the corpus callosum. Quantitative analysis in a 3-tesla MRI study revealed that hypoxia is associated with mild vasogenic cerebral edema irrespective of AMS. This finding is in keeping with case reports of suddenly symptomatic brain tumors and of cranial nerve palsies without AMS at high altitudes. Vasogenic edema may become cytotoxic (intracellular) in severe HACE.
FIGURE 476e-1 T2 magnetic resonance image of the brain of a patient with high-altitude cerebral edema (HACE) shows marked swelling and a hyperintense posterior body and splenium of the corpus callosum (area with dense opacity). The patient, a climber, went on to climb Mount Everest about 9 months after this episode of HACE. (With permission from Wilderness Environ Med 15:53–55, 2004.)
Impaired cerebral autoregulation in the presence of hypoxic cerebral vasodilation and altered permeability of the blood-brain barrier due to hypoxia-induced chemical mediators like histamine, arachidonic acid, and VEGF may all contribute to brain edema. In 1995, VEGF was first proposed as a potent promoter of capillary leakage in the brain at high altitude, and studies in mice have borne out this role. Although preliminary studies of VEGF in climbers have yielded inconsistent results regarding its association with altitude illness, indirect evidence of a role for this growth factor in AMS and HACE comes from the observation that dexamethasone, when used in the prevention and treatment of these conditions, blocks hypoxic upregulation of VEGF. Other factors in the development of cerebral edema may be the release of calcium-mediated nitric oxide and neuronally mediated adenosine, which may promote cerebral vasodilation.
Increased sympathetic activity triggered by hypoxia may also contribute to blood-brain barrier leakage. Enhanced optic-nerve sheath diameter with increasing severity of AMS has been noted and suggests an important role for increased intracranial pressures in the pathophysiology of AMS. Microhemorrhage formation caused by cytokines or damage through increased hydrostatic pressure is an important feature of HACE. Lesions in the globus pallidum (which is sensitive to hypoxia) leading to Parkinson’s disease have also been reported to be complications of HACE. Finally, the effect of hypoxia on reactive oxygen species and the role of these species in clinical AMS are unclear.
The pathophysiology of the most common and prominent symptom of AMS—headache—remains unclear because the brain itself is an insensate organ; only the meninges contain trigeminal sensory nerve fibers. The cause of high-altitude headache is multifactorial. Various chemicals and mechanical factors activate a final common pathway, the trigeminovascular system. In the genesis of high-altitude headache, the response to nonsteroidal anti-inflammatory drugs and glucocorticoids provides indirect evidence for involvement of the arachidonic acid pathway and inflammation. Although the International Headache Society acknowledges that high altitude may be a trigger for migraine, it is unclear whether high-altitude headache and migraine share the same pathophysiology.
Prevention and Treatment (Table 476e-1) Gradual ascent, with adequate time for acclimation, is the best method for the prevention of altitude illness. Even though there may be individual variation in the rate of acclimation, a graded ascent of ≤400 m from the previous day’s sleeping altitude is recommended above 3000 m, and taking every third day of gain in sleeping altitude as an extra day for acclimation is helpful. Spending one night at an intermediate altitude before proceeding to a higher altitude may enhance acclimation and attenuate the risk of AMS. Another protective factor in AMS is high-altitude exposure during the preceding 2 months; for example, the incidence and severity of AMS at 4300 m are reduced by 50% with an ascent after 1 week at an altitude of ≥2000 m rather than with an ascent from sea level. Studies have examined whether exposure to a normobaric hypoxic environment (in a room or a tent) before an ascent can provide protection against AMS. In double-blind placebo-controlled trials, repeated intermittent exposure (60–90 min) to normobaric hypoxia (up to 4500 m) or continuous exposure to 3000 m during 8 h of sleep for 7 consecutive days failed to reduce the incidence of AMS at altitudes of 4300–4559 m.
MANAGEMENT OF ALTITUDE ILLNESS |
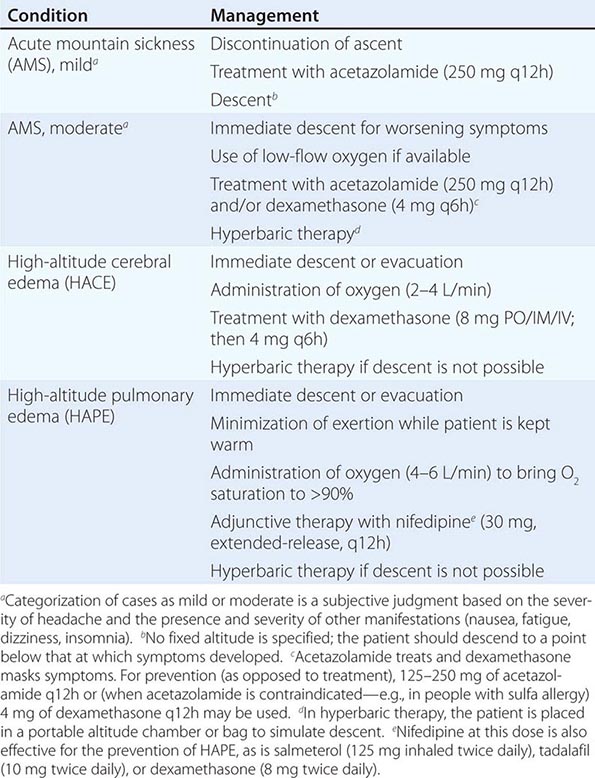
Clearly, a flexible itinerary that permits additional rest days will be helpful. Sojourners to high-altitude locations must be aware of the symptoms of altitude illness and should be encouraged not to ascend further if these symptoms develop. Any hint of HAPE (see below) or HACE mandates descent. Finally, proper hydration (but not overhydration) in high-altitude trekking and climbing, aimed at countering fluid loss due to hyperventilation and sweating, may also play a role in avoiding AMS. Pharmacologic prophylaxis at the time of travel to high altitudes is warranted for people with a history of AMS or when a graded ascent and acclimation are not possible—e.g., when rapid ascent is necessary for rescue purposes or when flight to a high-altitude location is required. Acetazolamide is the drug of choice for AMS prevention. It inhibits renal carbonic anhydrase, causing a prompt bicarbonate diuresis that leads to metabolic acidosis and hyperventilation. Acetazolamide (125–250 mg twice a day), administered for 1 day before ascent and continued for 2 or 3 days, is effective. Higher doses are not required. A meta-analysis limited to randomized controlled trials revealed that 125 mg of acetazolamide twice daily was effective in the prevention of AMS, with a relative-risk reduction of ~48% from values obtained with placebo. Paresthesia and a tingling sensation are common side effects of acetazolamide. This drug is a nonantibiotic sulfonamide that has low-level cross-reactivity with sulfa antibiotics; as a result, severe reactions are rare. Dexamethasone (8 mg/d in divided doses) is also effective. A large-scale, randomized, double-blind, placebo-controlled trial in partially acclimated trekkers has clearly shown that Ginkgo biloba is ineffective in the prevention of AMS. Recently, ibuprofen (600 mg three times daily) was shown to be beneficial in the prevention of AMS, but more definitive studies and proper gastrointestinal-bleeding risk assessment need to be conducted before ibuprofen can be routinely recommended for AMS prevention. Many drugs, including spironolactone, medroxyprogesterone, magnesium, calcium channel blockers, and antacids, confer no benefit in the prevention of AMS. Similarly, no efficacy studies are available for coca leaves (a weak form of cocaine), which are offered to high-altitude travelers in the Andes, or for soroche pills, which contain aspirin, caffeine, and acetaminophen and are sold over the counter in Bolivia and Peru. Finally, a word of caution applies in the pharmacologic prevention of altitude illness. A fast-growing population of climbers in pursuit of a summit are using prophylactic drugs such as glucocorticoids in an attempt to improve their performance; the outcome can be tragic because of potentially severe side effects of these drugs.
For the treatment of mild AMS, rest alone with analgesic use may be adequate. Descent and the use of acetazolamide and (if available) oxygen are sufficient to treat most cases of moderate AMS. Even a minor descent (400–500 m) may be adequate for symptom relief. For moderate AMS or early HACE, dexamethasone (4 mg orally or parenterally) is highly effective. For HACE, immediate descent is mandatory. When descent is not possible because of poor weather conditions or darkness, a simulation of descent in a portable hyperbaric chamber is effective and, like dexamethasone administration, “buys time.” Thus, in certain high-altitude locations (e.g., remote pilgrimage sites), the decision to bring along the light-weight hyperbaric chamber may prove life-saving. Like nifedipine, phosphodiesterase-5 inhibitors have no role in the treatment of AMS or HACE.
HIGH-ALTITUDE PULMONARY EDEMA
Risk Factors and Manifestations Unlike HACE (a neurologic disorder), HAPE is primarily a pulmonary problem and therefore is not necessarily preceded by AMS. HAPE develops within 2–4 days after arrival at high altitude; it rarely occurs after more than 4 or 5 days at the same altitude, probably because of remodeling and adaptation that render the pulmonary vasculature less susceptible to the effects of hypoxia. A rapid rate of ascent, a history of HAPE, respiratory tract infections, and cold environmental temperatures are risk factors. Men are more susceptible than women. People with abnormalities of the cardiopulmonary circulation leading to pulmonary hypertension—e.g., large patent foramen ovale, mitral stenosis, primary pulmonary hypertension, and unilateral absence of the pulmonary artery—are at increased risk of HAPE, even at moderate altitudes. For example, patent foramen ovale is four times more common among HAPE-susceptible individuals than in the general population. Echocardiography is recommended when HAPE develops at relatively low altitudes (<3000 m) and whenever cardiopulmonary abnormalities predisposing to HAPE are suspected.
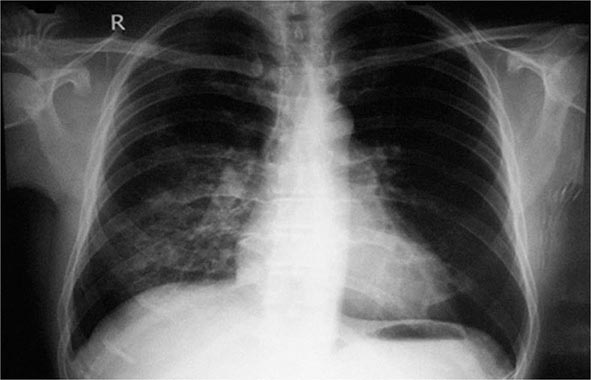
The initial manifestation of HAPE may be a reduction in exercise tolerance greater than that expected at the given altitude. Although a dry, persistent cough may presage HAPE and may be followed by the production of blood-tinged sputum, cough in the mountains is almost universal and the mechanism is poorly understood. Tachypnea and tachycardia, even at rest, are important markers as illness progresses. Crackles may be heard on auscultation but are not diagnostic. HAPE may be accompanied by signs of HACE. Patchy or localized opacities (Fig. 476e-2) or streaky interstitial edema may be noted on chest radiography. In the past, HAPE was mistaken for pneumonia due to the cold or for heart failure due to hypoxia and exertion. Kerley B lines or a bat-wing appearance are not seen on radiography. Electrocardiography may reveal right ventricular strain or even hypertrophy. Hypoxemia and respiratory alkalosis are consistently present unless the patient is taking acetazolamide, in which case metabolic acidosis may supervene. Assessment of arterial blood gases is not necessary in the evaluation of HAPE; an oxygen saturation reading with a pulse oximeter is generally adequate. The existence of a subclinical form of HAPE has been suggested by an increased alveolar-arterial oxygen gradient in Everest climbers near the summit, but hard evidence correlating this abnormality with the development of clinically relevant HAPE is lacking.
FIGURE 476e-2 Chest radiograph of a patient with high-altitude pulmonary edema shows opacity in the right middle and lower zones simulating pneumonic consolidation. The opacity cleared almost completely in 2 days with descent and supplemental oxygen.
Pathophysiology HAPE is a noncardiogenic pulmonary edema characterized by patchy pulmonary vasoconstriction that leads to overperfusion in some areas. This abnormality leads in turn to increased pulmonary capillary pressure (>18 mmHg) and capillary “stress” failure. The exact mechanism for the vasoconstriction is unknown. Endothelial dysfunction due to hypoxia may play a role by impairing the release of nitric oxide, an endothelium-derived vasodilator. At high altitude, HAPE-prone persons have reduced levels of exhaled nitric oxide. The effectiveness of phosphodiesterase-5 inhibitors in alleviating altitude-induced pulmonary hypertension, decreased exercise tolerance, and hypoxemia supports the role of nitric oxide in the pathogenesis of HAPE. One study demonstrated that prophylactic use of tadalafil, a phosphodiesterase-5 inhibitor, decreases the risk of HAPE by 65%. In contrast, the endothelium also synthesizes endothelin-1, a potent vasoconstrictor whose concentrations are higher than average in HAPE-prone mountaineers. Bosentan, an endothelin receptor antagonist, attenuates hypoxia-induced pulmonary hypertension, but further field studies with this drug are necessary.
Exercise and cold lead to increased pulmonary intravascular pressure and may predispose to HAPE. In addition, hypoxia-triggered increases in sympathetic drive may lead to pulmonary venoconstriction and extravasation into the alveoli from the pulmonary capillaries. Consistent with this concept, phentolamine, which elicits α-adrenergic blockade, improves hemodynamics and oxygenation in HAPE more than do other vasodilators. The study of tadalafil cited above also investigated dexamethasone in the prevention of HAPE. Surprisingly, dexamethasone reduced the incidence of HAPE by 78%—a greater decrease than with tadalafil. Besides possibly increasing the availability of endothelial nitric oxide, dexamethasone may have altered the excessive sympathetic activity associated with HAPE: the heart rate of participants in the dexamethasone arm of the study was significantly lowered. Finally, people susceptible to HAPE also display enhanced sympathetic activity during short-term hypoxic breathing at low altitudes.
Because many patients with HAPE have fever, peripheral leukocytosis, and an increased erythrocyte sedimentation rate, inflammation has been considered an etiologic factor in HAPE. However, strong evidence suggests that inflammation in HAPE is an epiphenomenon rather than the primary cause. Nevertheless, inflammatory processes (e.g., those elicited by viral respiratory tract infections) do predispose persons to HAPE—even those who are constitutionally resistant to its development.
Another proposed mechanism for HAPE is impaired transepithelial clearance of sodium and water from the alveoli. β-Adrenergic agonists upregulate the clearance of alveolar fluid in animal models. In a double-blind, randomized, placebo-controlled study of HAPE-susceptible mountaineers, prophylactic inhalation of the β-adrenergic agonist salmeterol reduced the incidence of HAPE by 50%. Other effects of β agonists may also contribute to the prevention of HAPE, but these findings are in keeping with the concept that alveolar fluid clearance may play a pathogenic role in this illness.
Prevention and Treatment (Table 476e-1) Allowing sufficient time for acclimation by ascending gradually (as discussed above for AMS and HACE) is the best way to prevent HAPE. Sustained-release nifedipine (30 mg), given once or twice daily, prevents HAPE in people who must ascend rapidly or who have a history of HAPE. Other drugs for the prevention of HAPE are listed in Table 476e-1 (footnote e). Although dexamethasone is listed for prevention, its adverse-effect profile requires close monitoring. Acetazolamide has been shown to blunt hypoxic pulmonary vasoconstriction in animal models, and this observation warrants further study in HAPE prevention. However, one large study failed to show a decrease in pulmonary vasoconstriction in partially acclimated individuals given acetazolamide.
Early recognition is paramount in the treatment of HAPE, especially when it is not preceded by the AMS symptoms of headache and nausea. Fatigue and dyspnea at rest may be the only initial manifestations. Descent and the use of supplementary oxygen (aimed at bringing oxygen saturation to >90%) are the most effective therapeutic interventions. Exertion should be kept to a minimum, and the patient should be kept warm. Hyperbaric therapy in a portable altitude chamber may be used if descent is not possible and oxygen is not available. Oral sustained-release nifedipine (30 mg once or twice daily) can be used as adjunctive therapy. Inhaled β agonists, which are safe and convenient to carry, are useful in the prevention of HAPE and may be effective in its treatment, although no trials have yet been carried out. Inhaled nitric oxide and expiratory positive airway pressure may also be useful therapeutic measures but may not be available in high-altitude settings. No studies have investigated phosphodiesterase-5 inhibitors in the treatment of HAPE, but reports have described their use in clinical practice. The mainstays of treatment remain descent and (if available) oxygen.
In AMS, if symptoms abate (with or without acetazolamide), the patient may reascend gradually to a higher altitude. Unlike that in acute respiratory distress syndrome (another noncardiogenic pulmonary edema), the architecture of the lung in HAPE is usually well preserved, with rapid reversibility of abnormalities (Fig. 476e-2). This fact has allowed some people with HAPE to reascend slowly after a few days of descent and rest. In HACE, reascent after a few days may not be advisable during the same trip.
OTHER HIGH-ALTITUDE PROBLEMS
Sleep Impairment The mechanisms underlying sleep problems, which are among the most common adverse reactions to high altitude, include increased periodic breathing; changes in sleep architecture, with increased time in lighter sleep stages; and changes in rapid eye movement sleep. Sojourners should be reassured that sleep quality improves with acclimation. In cases where drugs do need to be used, acetazolamide (125 mg before bedtime) is especially useful because this agent decreases hypoxemic episodes and alleviates sleeping disruptions caused by excessive periodic breathing. Whether combining acetazolamide with temazepam or zolpidem is more effective than administering acetazolamide alone is unknown. In combinations, the doses of temazepam and zolpidem should not be increased by >10 mg at high altitudes. Limited evidence suggests that diazepam causes hypoventilation at high altitudes and therefore is contraindicated. For trekkers with obstructive sleep apnea who are using a continuous positive airway pressure (CPAP) machine, the addition of acetazolamide, which will decrease centrally mediated sleep apnea, may be helpful. There is evidence to show that obstructive sleep apnea at high altitude may decrease and “convert” to central sleep apnea.
Gastrointestinal Issues High-altitude exposure may be associated with increased gastric and duodenal bleeding, but further studies are required to determine whether there is a causal effect. Because of decreased atmospheric pressure and consequent intestinal gas expansion at high altitudes, many sojourners experience abdominal bloating and distension as well as excessive flatus expulsion. In the absence of diarrhea, these phenomena are normal, if sometimes uncomfortable. Accompanying diarrhea, however, may indicate the involvement of bacteria or Giardia parasites, which are common at many high-altitude locations in the developing world. Prompt treatment with fluids and empirical antibiotics may be required to combat dehydration in the mountains. Finally, hemorrhoids are common on high-altitude treks; treatment includes hot soaks, application of hydrocortisone ointment, and measures to avoid constipation.
High-Altitude Cough High-altitude cough can be debilitating and is sometimes severe enough to cause rib fracture, especially at >5000 m. The etiology is probably multifactorial. Although high-altitude cough has been attributed to inspiration of cold dry air, this explanation appears not to be sufficient by itself; in long-duration studies in hypobaric chambers, cough has occurred despite controlled temperature and humidity. The implication is that hypoxia also plays a role. Exercise can precipitate cough at high altitudes, possibly because of water loss from the respiratory tract. Long-acting β agonists and glucocorticoids prevent bronchoconstriction that otherwise may be brought on by cold and exercise. In general, infection does not seem to be a common etiology. Anecdotal reports have described the efficacy of an inhaled combination of fluticasone and salmeterol in the treatment of high-altitude cough; however, a placebo-controlled, randomized trial failed to support this beneficial effect. Furthermore, anecdotal evidence supports the utility of the proton pump inhibitor omeprazole in preventing gastroesophageal reflux in some trekkers and climbers. In most situations, cough resolves upon descent.
High-Altitude Neurologic Events Unrelated to “Altitude Illness” Transient ischemic attacks (TIAs) and strokes have been well described in high-altitude sojourners outside the setting of altitude sickness. However, these descriptions are not based on cause (hypoxia) and effect. In general, symptoms of AMS present gradually, whereas many of these neurologic events happen suddenly. The population that suffers strokes and TIAs at sea level is generally an older age group with other risk factors, whereas those so afflicted at high altitudes are generally younger and probably have fewer risk factors for atherosclerotic vascular disease. Other mechanisms (e.g., migraine, vasospasm, focal edema, hypocapneic vasoconstriction, hypoxia in the watershed zones of minimal cerebral blood flow, or cardiac right-to-left shunt) may be operative in TIAs and strokes at high altitude.
Subarachnoid hemorrhage, transient global amnesia, delirium, and cranial nerve palsies (e.g., lateral rectus palsy) occurring at high altitudes but outside the setting of altitude sickness have also been well described. Syncope is common at moderately high altitudes, generally occurs shortly after ascent, usually resolves without descent, and appears to be a vasovagal event related to hypoxemia. Seizures occur rarely with HACE, but hypoxemia and hypocapnia, which are prevalent at high altitudes, are well-known triggers that may contribute to new or breakthrough seizures in predisposed individuals. Nevertheless, the consensus among experts is that sojourners with well-controlled seizure disorders can ascend to high altitudes. Ophthalmologic problems, such as cortical blindness, amaurosis fugax, and retinal hemorrhage with macular involvement and compromised vision, are well recognized. Visual problems from previous refractive surgery and blurred monocular vision—due either to the use of a transdermal scopolamine patch (touching the eye after touching the patch) or to dry-eye syndrome—may also occur in the field at high altitudes and may be confused with neurologic conditions. Finally, persons with hypercoagulable conditions (e.g., antiphospholipid syndrome, protein C deficiency) who are asymptomatic at sea level may experience cerebral venous thrombosis (possibly due to the enhanced blood viscosity triggered by polycythemia and dehydration) at high altitudes. Proper history taking, examination, and prompt investigations where possible will help define these conditions as entities separate from altitude sickness. Administration of oxygen (where available) and prompt descent are the cornerstones of treatment of most of these neurologic conditions.
Psychological/Psychiatric Problems Delirium characterized by a sudden change in mental status, a short attention span, disorganized thinking, and an agitated state during the period of confusion has been well described in mountain climbers and trekkers without a prior history. In addition, anxiety attacks, often triggered at night by excessive periodic breathing, are well documented. The contribution of hypoxia to these conditions is unknown. Expedition medical kits need to include antipsychotic injectable drugs to control psychosis in patients in remote high-altitude locations.
PREEXISTING MEDICAL ISSUES
Because travel to high altitudes is increasingly popular, common conditions such as hypertension, coronary artery disease, and diabetes are more frequently encountered among high-altitude sojourners. This situation is of particular concern for the thousands of elderly pilgrims with medical problems who visit high-altitude sacred areas (e.g., in the Himalayas) each year. In recent years, high-altitude travel has attracted intrepid trekkers who are taking immunosuppressive medications (e.g., kidney transplant recipients or patients undergoing chemotherapy). Recommended vaccinations and other precautions (e.g., hand washing) may be especially important for this group. Although most of these medical conditions do not appear to influence susceptibility to altitude illness, they may be exacerbated by ascent to altitude, exertion in cold conditions, and hypoxemia. Advice regarding the advisability of high-altitude travel and the impact of high-altitude hypoxia on these preexisting conditions is becoming increasingly relevant, but there are no evidence-based guidelines. In addition, recommendations made for relatively low altitudes (~3000 m) may not hold true for higher altitudes (>4000 m), where hypoxic stress is greater. Personal risks and benefits must be clearly thought through before ascent.
Hypertension At high altitudes, enhanced sympathetic activity may lead to a transient rise in blood pressure. Occasionally, nonhypertensive, healthy, asymptomatic trekkers have pathologically high blood pressure at high altitude that rapidly normalizes without medicines on descent. Sojourners should continue to take their antihypertensive medications at high altitudes. Hypertensive patients are not more likely than others to develop altitude illness. Because the probable mechanism of high-altitude hypertension is α-adrenergic activity, anti-α-adrenergic drugs like prazosin have been suggested for symptomatic patients and those with labile hypertension. It is best to start taking the drug several weeks before the trip and to carry a sphygmomanometer if a trekker has labile hypertension. Sustained-release nifedipine may also be useful. For a common problem like hypertension, there is clearly inadequate knowledge on which to base appropriate recommendations.
Coronary Artery Disease Myocardial oxygen demand and maximal heart rate are reduced at high altitudes because the VO2 max (maximal oxygen consumption) decreases with increasing altitude. This effect may explain why signs of cardiac ischemia or dysfunction usually are not seen in healthy persons at high altitudes. Asymptomatic, fit individuals with no risk factors need not undergo any tests for coronary artery disease before ascent. For persons with ischemic heart disease, previous myocardial infarction, angioplasty, and/or bypass surgery, an exercise treadmill test is indicated. A strongly positive treadmill test is a contraindication for high-altitude trips. Patients with poorly controlled arrhythmias should avoid high-altitude travel, but patients with arrhythmias that are well controlled with antiarrhythmic medications do not seem to be at increased risk. Sudden cardiac deaths are not noted with a greater frequency in the Alps than at lower altitudes; although sudden cardiac deaths are encountered every trekking season in the higher Himalayan range, accurate documentation is lacking.
Asthma Although cold air and exercise may provoke acute bronchoconstriction, asthmatic patients usually have fewer problems at high than at low altitudes, possibly because of decreased allergen levels and increased circulating catecholamine levels. Nevertheless, asthmatic individuals should carry all their medications, including oral glucocorticoids, with proper instructions for use in case of an exacerbation. Severely asthmatic persons should be cautioned against ascending to high altitudes.
Pregnancy In general, low-risk pregnant women ascending to 3000 m are not at special risk except for the relative unavailability of medical care in many high-altitude locations, especially in developing countries. Despite the lack of firm data on this point, venturing higher than 3000 m to altitudes at which oxygen saturation drops steeply seems unadvisable for pregnant women.
Obesity Although living at a high altitude has been suggested as a means of controlling obesity, obesity has also been reported to be a risk factor for AMS, probably because nocturnal hypoxemia is more pronounced in obese individuals. Hypoxemia may also lead to greater pulmonary hypertension, thus possibly predisposing the trekker to HAPE.
Sickle Cell Disease High altitude is one of the rare environmental exposures that occasionally provokes a crisis in persons with the sickle cell trait. Even when traversing mountain passes as low as 2500 m, people with sickle cell disease have been known to have a vasoocclusive crisis. Sickle cell disease needs to be considered when persons traveling to high altitudes become unwell and develop left-upper-quadrant pain. Patients with known sickle cell disease who need to travel to high altitudes should use supplemental oxygen and travel with caution.
Diabetes Mellitus Trekking at high altitudes may enhance sugar uptake. Thus, high-altitude travel may not pose problems for persons with diabetes that is well controlled with oral hypoglycemic agents. An eye examination before travel may be useful. Patients taking insulin may require lower doses on trekking/climbing days than on rest days. Because of these variations, diabetic patients need to carry a reliable glucometer and use fast-acting insulin. Ready access to sweets is also essential. It is important for companions of diabetic trekkers to be fully aware of potential problems like hypoglycemia.
Chronic Lung Disease Depending on disease severity and access to medical care, preexisting lung disease may not always preclude high-altitude travel. A proper pretravel evaluation must be conducted. Supplemental oxygen may be required if the predicted PaO2 for the altitude is <50–55 mmHg. Preexisting pulmonary hypertension may also need to be assessed in these patients. If the result is positive, patients should be discouraged from ascending to high altitudes; if such travel is necessary, treatment with sustained-release nifedipine (20 mg twice a day) should be considered. Small-scale studies have revealed that when patients with bullous disease reach ~5000 m, bullous expansion and pneumothorax are not noted. Compared with information on chronic obstructive pulmonary disease, fewer data exist about the safety of travel to high altitude for people with pulmonary fibrosis, but acute exacerbation of pulmonary fibrosis has been seen at high altitude. A handheld pulse oximeter can be useful to check for oxygen saturation.
Chronic Kidney Disease Patients with chronic kidney disease can tolerate short-term stays at high altitudes, but theoretical concern persists about progression to end-stage renal disease. Acetazolamide, the drug most commonly used for altitude sickness, should be avoided by anyone with preexisting metabolic acidosis, which can be exacerbated by this drug. In addition, the acetazolamide dosage should be adjusted when the glomerular filtration rate falls below 50 mL/min, and the drug should not be used at all if this value falls below 10 mL/min.
CHRONIC MOUNTAIN SICKNESS AND HIGH-ALTITUDE PULMONARY HYPERTENSION
Chronic mountain sickness (Monge’s disease) is a disease of long-term residents of altitudes above 2500 m that is characterized by excessive erythrocytosis with moderate to severe pulmonary hypertension leading to cor pulmonale. This condition was originally described in South America and has also been documented in Colorado and in the Han Chinese population in Tibet. Migration to a low altitude results in the resolution of chronic mountain illness. Venesection and acetazolamide are helpful.
High-altitude pulmonary hypertension is also a subacute disease of long-term high-altitude residents. Unlike Monge’s disease, this syndrome is characterized primarily by pulmonary hypertension (not erythrocytosis) leading to heart failure. Indian soldiers living at extreme altitudes for prolonged periods and Han Chinese infants born in Tibet have presented with the adult and infantile forms, respectively. High-altitude pulmonary hypertension bears a striking pathophysiologic resemblance to brisket disease in cattle. Descent to a lower altitude is curative.
477e | Hyperbaric and Diving Medicine |
WHAT IS HYPERBARIC AND DIVING MEDICINE?
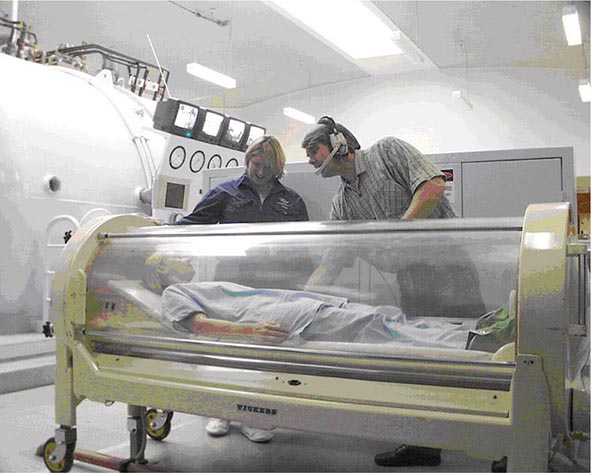
Hyperbaric medicine is the treatment of health disorders using whole-body exposure to pressures greater than 101.3 kPa (1 atmosphere or 760 mmHg). In practice, this almost always means the administration of hyperbaric oxygen therapy (HBO2T). The Undersea and Hyperbaric Medical Society (UHMS) defines HBO2T as: “a treatment in which a patient breathes 100% oxygen … while inside a treatment chamber at a pressure higher than sea level pressure (i.e., >1 atmosphere absolute or ATA).” The treatment chamber is an airtight vessel variously called a hyperbaric chamber, recompression chamber, or decompression chamber, depending on the clinical and historical context. Such chambers may be capable of compressing a single patient (a monoplace chamber) or multiple patients and attendants as required (a multiplace chamber) (Figs. 477e-1 and 477e-2). Historically, these compression chambers were first used for the treatment of divers and compressed air workers suffering decompression sickness (DCS; “the bends”). Although the prevention and treatment of disorders arising after decompression in diving, aviation, and space flight has developed into a specialized field of its own, it remains closely linked to the broader practice of hyperbaric medicine.
FIGURE 477e-1 A monoplace chamber. (Prince of Wales Hospital, Sydney.)
FIGURE 477e-2 A chamber designed to treat multiple patients. (Karolinska University Hospital.)
Despite an increased understanding of mechanisms and an improving evidence basis, hyperbaric medicine has struggled to achieve widespread recognition as a “legitimate” therapeutic measure. There are several contributing factors, but high among them are a poor grounding in general oxygen physiology and oxygen therapy at medical schools and a continuing tradition of charlatans advocating hyperbaric therapy (often using air) as a panacea. Funding for both basic and clinical research has been difficult in an environment where the pharmacologic agent under study is abundant, cheap, and unpatentable. Recently, however, there are signs of an improved appreciation of the potential importance of HBO2T with significant National Institutes of Health (NIH) funding for mechanisms research and from the U.S. military for clinical investigation.
MECHANISMS OF HYPERBARIC OXYGEN
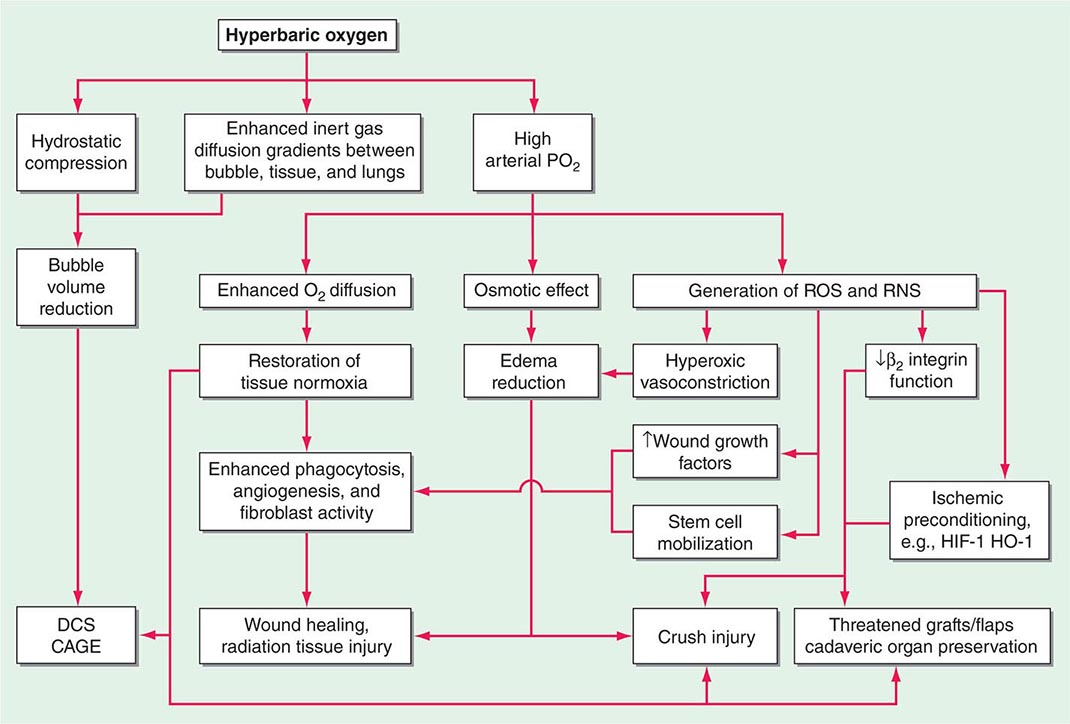
Increased hydrostatic pressure will reduce the volume of any bubbles present within the body (see “Diving Medicine”), and this is partly responsible for the success of prompt recompression in DCS and arterial gas embolism. Supplemental oxygen breathing has a dose-dependent effect on oxygen transport, ranging from improvement in hemoglobin oxygen saturation when a few liters per minute are delivered by simple mask at 101.3 kPa (1 ATA) to raising the dissolved plasma oxygen sufficiently to sustain life without the need for hemoglobin at all when 100% oxygen is breathed at 303.9 kPa (3 ATA). Most HBO2T regimens involve oxygen breathing at between 202.6 kPa and 283.6 kPa (2 and 2.8 ATA), and the resultant increase in arterial oxygen tensions to >133.3 kPa (1000 mmHg) has widespread physiologic and pharmacologic consequences (Fig. 477e-3).
FIGURE 477e-3 Mechanisms of action of hyperbaric oxygen. There are many consequences of compression and oxygen breathing. The cell-signaling effects of HBO2T are the least understood but potentially most important. Examples of indications for use are shown in the shaded boxes. CAGE, cerebral arterial gas embolism; DCS, decompression sickness; HIF-1, hypoxia inducible factor-1; HO-1, hemoxygenase 1; RNS, reactive nitrogen species; ROS, reactive oxygen species.
One direct consequence of such high intravascular tension is to increase greatly the effective capillary-tissue diffusion distance for oxygen such that oxygen-dependent cellular processes can resume in hypoxic tissues. Important as this may be, the mechanism of action is not limited to this restoration of oxygenation in hypoxic tissue. Indeed, there are pharmacologic effects that are profound and long-lasting. Although removal from the hyperbaric chamber results in a rapid return of poorly vascularized tissues to their hypoxic state, even a single dose of HBO2T produces changes in fibroblast, leukocyte, and angiogenic functions and antioxidant defenses that persist many hours after oxygen tensions are returned to pretreatment levels.
It is widely accepted that oxygen in high doses produces adverse effects due to the production of reactive oxygen species (ROS) such as superoxide (O2–) and hydrogen peroxide (H2O2). It has become increasingly clear over the last decade that both ROS and reactive nitrogen species (RNS) such as nitric oxide (NO) participate in a wide range of intracellular signaling pathways involved in the production of a range of cytokines, growth factors, and other inflammatory and repair modulators. Such mechanisms are complex and at times apparently paradoxical. For example, when used to treat chronic hypoxic wounds, HBO2T has been shown to enhance the clearance of cellular debris and bacteria by providing the substrate for macrophage phagocytosis; stimulate growth factor synthesis by increased production and stabilization of hypoxia-inducible factor 1 (HIF-1); inhibit leukocyte activation and adherence to damaged endothelium; and mobilize CD34+ pluripotent vasculogenic progenitor cells from the bone marrow. The interactions between these mechanisms remain a very active field of investigation. One exciting development is the concept of hyperoxic preconditioning in which a short exposure to HBO2 can induce tissue protection against future hypoxic/ischemic insult, most likely through an inhibition of mitochondrial permeability transition pore (MPTP) opening and the release of cytochrome c. By targeting these mechanisms of cell death during reperfusion events, HBO2 has potential applications in a variety of settings including organ transplantation. One randomized clinical trial suggested that HBO2T prior to coronary artery bypass grafting reduces biochemical markers of ischemic stress and improves neurocognitive outcomes.
ADVERSE EFFECTS OF THERAPY
HBO2T is generally well tolerated and safe in clinical practice. Adverse effects are associated with both alterations in pressure (barotrauma) and the administration of oxygen.
BAROTRAUMA
Barotrauma occurs when any noncompliant gas-filled space within the body does not equalize with environmental pressure during compression or decompression. About 10% of patients complain of some difficulty equalizing middle-ear pressure early in compression, and although most of these problems are minor and can be overcome with training, 2–5% of conscious patients require middle-ear ventilation tubes or formal grommets across the tympanic membrane. Unconscious patients cannot equalize and should have middle-ear ventilation tubes placed prior to compression if possible. Other less common sites for barotrauma of compression include the respiratory sinuses and dental caries. The lungs are potentially vulnerable to barotrauma of decompression as described below in the section on diving medicine, but the decompression following HBO2T is so slow that pulmonary gas trapping is extremely rare in the absence of an undrained pneumothorax or lesions such as bullae.
OXYGEN TOXICITY
The practical limit to the dose of oxygen, either in a single treatment session or in a series of daily sessions, is oxygen toxicity. The most common acute manifestation is a seizure, often preceded by anxiety and agitation, during which time a switch from oxygen to air breathing may avoid the convulsion. Hyperoxic seizures are typically generalized tonic-clonic seizures followed by a variable postictal period. The cause is an overwhelming of the antioxidant defense systems within the brain. Although clearly dose-dependent, onset is very variable both between individuals and within the same individual on different days. In routine clinical hyperbaric practice, the incidence is about 1:1500 to 1:2000 compressions.
Chronic oxygen poisoning most commonly manifests as myopic shift. This is due to alterations in the refractive index of the lens following oxidative damage that reduces the solubility of lenticular proteins in a process similar to that associated with senescent cataract formation. Up to 75% of patients show deterioration in visual acuity after a course of 30 treatments at 202.6 kPa (2 ATA). Although most return to pretreatment values 6–12 weeks after cessation of treatment, a small proportion do not recover. A more rapid maturation of preexisting cataracts has occasionally been associated with HBO2T. Although a theoretical problem, the development of pulmonary oxygen toxicity over time does not seem to be problematic in practice—probably due to the intermittent nature of the exposure.
CONTRAINDICATIONS TO HYPERBARIC OXYGEN
There are few absolute contraindications to HBO2T. The most commonly encountered is an untreated pneumothorax. A pneumothorax may expand rapidly on decompression and come under tension. Prior to any compression, patients with a pneumothorax should have a patent chest drain in place. The presence of other obvious risk factors for pulmonary gas trapping such as bullae should trigger a very cautious analysis of the risks of treatment versus benefit. Prior bleomycin treatment deserves special mention because of its association with a partially dose-dependent pneumonitis in about 20% of people. These individuals appear to be at particular risk for rapid deterioration of ventilatory function following exposure to high oxygen tensions. The relationship between distant bleomycin exposure and subsequent risk of pulmonary oxygen toxicity is uncertain, however late pulmonary fibrosis is a potential complication of bleomycin, and any patient with a history of receiving this drug should be carefully counseled prior to exposure to HBO2T. For those recently exposed to doses above 300,000 IU (200 mg) and whose course was complicated by a respiratory reaction to bleomycin, compression should be avoided except in a life-threatening situation.
INDICATIONS FOR HYPERBARIC OXYGEN
The appropriate indications for HBO2T are controversial and evolving. Practitioners in this area are in an unusual position. Unlike most branches of medicine, hyperbaric physicians do not deal with a range of disorders within a defined organ system (e.g., cardiology), nor are they masters of a therapy specifically designed for a single category of disorders. Inevitably, the encroachment of hyperbaric physicians into other medical fields generates suspicion from specialist practitioners in those fields. At the same time this relatively benign therapy, the prescription and delivery of which requires no medical license in most jurisdictions (including the United States), attracts both charlatans and well-motivated proselytizers who tout the benefits of oxygen for a plethora of chronic incurable diseases. This battle on two fronts has meant that mainstream hyperbaric physicians have been particularly careful to claim effectiveness only for those conditions where there is a reasonable body of supporting evidence.
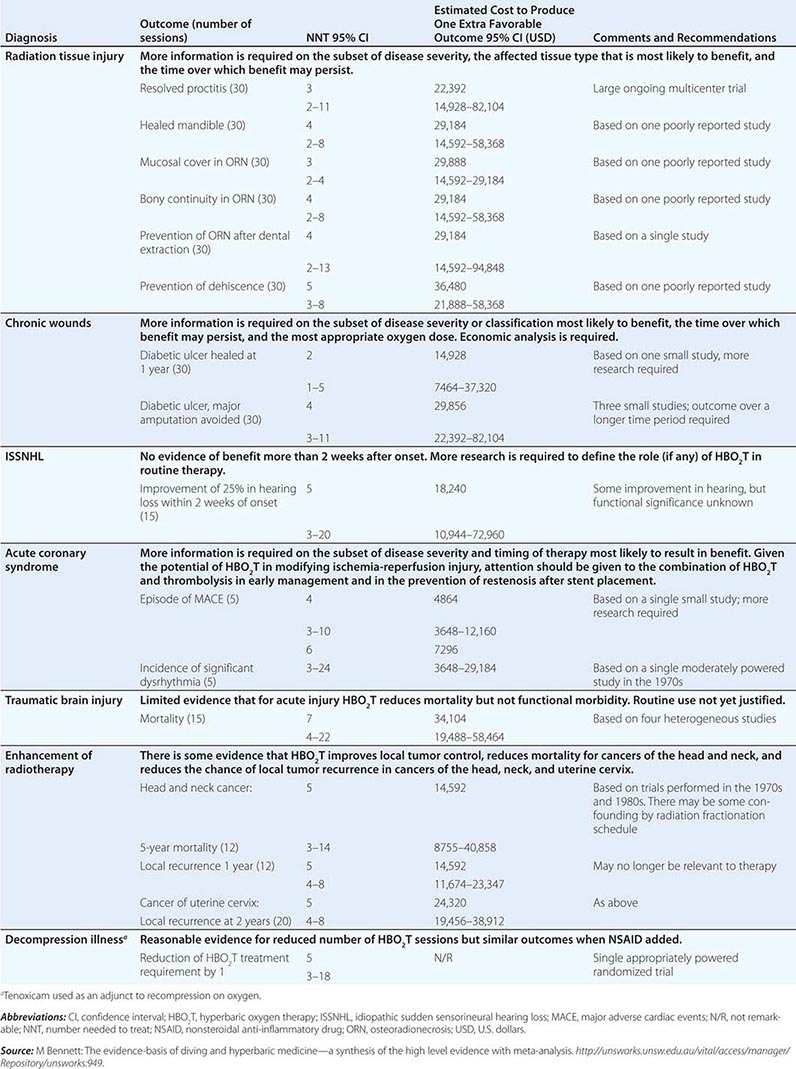
In 1977, the UHMS systematically examined claims for the use of HBO2T in more than 100 disorders and found sufficient evidence to support routine use in only 12. The Hyperbaric Oxygen Therapy Committee of that organization has continued to update this list periodically with an increasingly formalized system of appraisal for new indications and emerging evidence (Table 477e-1). Around the world, other relevant medical organizations have generally taken a similar approach, although indications vary considerably—particularly those recommended by hyperbaric medical societies in Russia and China where HBO2T has gained much wider support than in the United States, Europe, and Australasia. Recently, several Cochrane reviews have examined the randomized trial evidence for many putative indications, including attempts to examine the cost-effectiveness of HBO2T. Table 477e-2 is a synthesis of these two approaches and lists the estimated cost of attaining health outcomes with the use of HBO2T. Any savings associated with alternative treatment strategies avoided as a result of HBO2T are not accounted for in these estimates (e.g., the avoidance of lower leg amputation in diabetic foot ulcers). Following are short reviews of three important indications currently accepted by the UHMS.
CURRENT LIST OF INDICATIONS FOR HYPERBARIC OXYGEN THERAPY |
Source: The Undersea and Hyperbaric Medical Society (2013).
SELECTED INDICATIONS FOR WHICH THERE IS PROMISING EFFICACY FOR THE APPLICATION OF HYPERBARIC OXYGEN THERAPY |
LATE RADIATION TISSUE INJURY
Radiotherapy is a well-established treatment for suitable malignancies. In the United States alone, approximately 300,000 individuals annually will become long-term survivors of cancer treated by irradiation. Serious radiation-related complications developing months or years after treatment (late radiation tissue injury [LRTI]) will significantly affect between 5 and 15% of those long-term survivors, although incidence varies widely with dose, age, and site. LRTI is most common in the head and neck, chest wall, breast, and pelvis.
Pathology and Clinical Course With time, tissues undergo a progressive deterioration characterized by a reduction in the density of small blood vessels (reduced vascularity) and the replacement of normal tissue with dense fibrous tissue (fibrosis). An alternative model of pathogenesis suggests that rather than a primary hypoxia, the principle trigger is an overexpression of inflammatory cytokines that promote fibrosis, probably through oxidative stress and mitochondrial dysfunction, and a secondary tissue hypoxia. Ultimately, and often triggered by a further physical insult such as surgery or infection, there may be insufficient oxygen to sustain normal function, and the tissue becomes necrotic (radiation necrosis). LRTI may be life-threatening and significantly reduce quality of life. Historically, the management of these injuries has been unsatisfactory. Conservative treatment is usually restricted to symptom management, whereas definitive treatment traditionally entails surgery to remove the affected part and extensive repair. Surgical intervention in an irradiated field is often disfiguring and associated with an increased incidence of delayed healing, breakdown of a surgical wound, or infection. HBO2T may act by several mechanisms to improve this situation, including edema reduction, vasculogenesis, and enhancement of macrophage activity (Fig. 477e-3). The intermittent application of HBO2 is the only intervention shown to increase the microvascular density in irradiated tissue.
Clinical Evidence The typical course of HBO2T consists of 30 once-daily compressions to 202.6–243.1 kPa (2–2.4 ATA) for 1.5–2 h each session, often bracketed around surgical intervention if required. Although HBO2T has been used for LRTI since at least 1975, most clinical studies have been limited to small case series or individual case reports. In a review, Feldmeier and Hampson located 71 such reports involving a total of 1193 patients across eight different tissues. There were clinically significant improvements in the majority of patients, and only 7 of 71 reports indicated a generally poor response to HBO2T. A Cochrane systematic review with meta-analysis included six randomized trials published since 1985 and drew the following conclusions (see Table 477e-2 for numbers needed to treat): HBO2T improves healing in radiation proctitis (relative risk [RR] of healing with HBO2T 2.7; 95% confidence interval [CI] 1.2–6) and after hemimandibulectomy and reconstruction of the mandible (RR 1.4; 95% CI 1.1–1.8); HBO2T improves the probability of achieving mucosal coverage (RR 1.4; 95% CI 1.2–1.6) and the restoration of bony continuity with osteoradionecrosis (ORN) (RR 1.4; 95% CI 1.1–1.8); HBO2T prevents the development of ORN following tooth extraction from a radiation field (RR 1.4; 95% CI 1.08–1.7) and reduces the risk of wound dehiscence following grafts and flaps in the head and neck (RR 4.2; 95% CI 1.1–16.8). Conversely, there was no evidence of benefit in established radiation brachial plexus lesions or brain injury.
SELECTED PROBLEM WOUNDS
A problem wound is any cutaneous ulceration that requires a prolonged time to heal, does not heal, or recurs. In general, wounds referred to hyperbaric facilities are those where sustained attempts to heal by other means have failed. Problem wounds are common and constitute a significant health problem. It has been estimated that 1% of the population of industrialized countries will experience a leg ulcer at some time. The global cost of chronic wound care may be as high as U.S. $25 billion per year.
Pathology and Clinical Course By definition, chronic wounds are indolent or progressive and resistant to the wide array of treatments applied. Although there are many contributing factors, most commonly these wounds arise in association with one or more comorbidities such as diabetes, peripheral venous or arterial disease, or prolonged pressure (decubitus ulcers). First-line treatments are aimed at correction of the underlying pathology (e.g., vascular reconstruction, compression bandaging, or normalization of blood glucose level), and HBO2T is an adjunctive therapy to good general wound care practice to maximize the chance of healing.
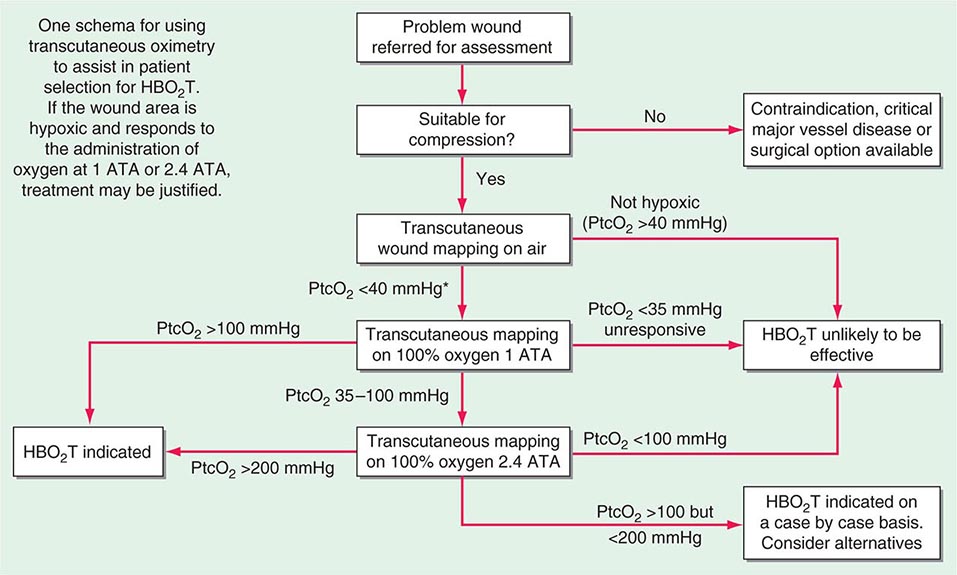
For most indolent wounds, hypoxia is a major contributor to failure to heal. Many guidelines to patient selection for HBO2T include the interpretation of transcutaneous oxygen tensions around the wound while breathing air and oxygen at pressure (Fig. 477e-4). Wound healing is a complex and incompletely understood process. While it appears that in acute wounds healing is stimulated by the initial hypoxia, low pH, and high lactate concentrations found in freshly injured tissue, some elements of tissue repair are extremely oxygen dependent, for example, collagen elaboration and deposition by fibroblasts, and bacterial killing by macrophages. In this complicated interaction between wound hypoxia and peri-wound oxygenation, successful healing relies on adequate tissue oxygenation in the area surrounding the fresh wound. Certainly, wounds that lie in hypoxic tissue beds are those that most often display poor or absent healing. Some causes of tissue hypoxia will be reversible with HBO2T, whereas some will not (e.g., in the presence of severe large vessel disease). When tissue hypoxia can be overcome by a high driving pressure of oxygen in the arterial blood, this can be demonstrated by measuring the tissue partial pressure of oxygen using an implantable oxygen electrode or, more commonly, a modified transcutaneous Clarke electrode.
FIGURE 477e-4 Determining suitability for hyperbaric oxygen therapy (HBO2T) guided by transcutaneous oximetry around the wound bed. *In diabetic patients, <50 mmHg may be more appropriate. PtcO2, transcutaneous oxygen pressure.
The intermittent presentation of oxygen to those hypoxic tissues facilitates a resumption of healing. These short exposures to high oxygen tensions have long-lasting effects (at least 24 h) on a wide range of healing processes (Fig. 477e-3). The result is a gradual improvement in oxygen tension around the wound that reaches a plateau in experimental studies at about 20 treatments over 4 weeks. Improvements in oxygenation are associated with an eight- to ninefold increase in vascular density over both normobaric oxygen and air-breathing controls.
Clinical Evidence The typical course of HBO2T consists of 20–30 once-daily compressions to 2–2.4 ATA for 1.5–2 h each session, but is highly dependent on the clinical response. There are many case series in the literature supporting the use of HBO2T for a wide range of problem wounds. Both retrospective and prospective cohort studies suggest that 6 months after a course of therapy, about 70% of indolent ulcers will be substantially improved or healed. Often these ulcers have been present for many months or years, suggesting that the application of HBO2T has a profound effect, either primarily or as a facilitator of other strategies. A recent Cochrane review included nine randomized controlled trials (RCTs) and concluded that the chance of ulcer healing improved about fivefold with HBO2T (RR 5.20; 95% CI 1.25–21.66; p = .02). Although there was a trend to benefit with HBO2T, there was no statistically significant difference in the rate of major amputations (RR 0.36; 95% CI 0.11–1.18).
CARBON MONOXIDE POISONING
Carbon monoxide (CO) is a colorless, odorless gas formed during incomplete hydrocarbon combustion. Although CO is an essential endogenous neurotransmitter linked to NO metabolism and activity, it is also a leading cause of poisoning death, and in the United States alone results in more than 50,000 emergency department visits per year and about 2000 deaths. Although there are large variations from country to country, about half of nonlethal exposures are due to self-harm. Accidental poisoning is commonly associated with defective or improperly installed heaters, house fires, and industrial exposures. The motor vehicle is by far the most common source of intentional poisoning.
Pathology and Clinical Course The pathophysiology of carbon monoxide exposure is incompletely understood. CO binds to hemoglobin with an affinity more than 200 times that of oxygen, directly reducing the oxygen-carrying capacity of blood, and further promoting tissue hypoxia by shifting the oxyhemoglobin dissociation curve to the left. CO is also an anesthetic agent that inhibits evoked responses and narcotizes experimental animals in a dose-dependent manner. The associated loss of airway patency together with reduced oxygen carriage in blood may cause death from acute arterial hypoxia in severe poisoning. CO may also cause harm by other mechanisms including direct disruption of cellular oxidative processes, binding to myoglobin and hepatic cytochromes, and peroxidation of brain lipids.
The brain and heart are the most sensitive target organs due to their high blood flow, poor tolerance of hypoxia, and high oxygen requirements. Minor exposure may be asymptomatic or present with vague constitutional symptoms such as headache, lethargy, and nausea, whereas higher doses may present with poor concentration and cognition, short-term memory loss, confusion, seizures, and loss of consciousness. While carboxyhemoglobin (COHb) levels on admission do not necessarily reflect the severity or the prognosis of CO poisoning, cardiorespiratory arrest carries a very poor prognosis. Over the longer term, surviving patients commonly have neuropsychological sequelae. Motor disturbances, peripheral neuropathy, hearing loss, vestibular abnormalities, dementia, and psychosis have all been reported. Risk factors for poor outcome are age >35 years, exposure for >24 h, acidosis, and loss of consciousness.
Clinical Evidence The typical course of HBO2T consists of two to three compressions to 2–2.8 ATA for 1.5–2 h each session. It is common for the first two compressions to be delivered within 24 h of the exposure. CO poisoning is one of the longest-standing indications for HBO2T—based largely on the obvious connection between exposure, tissue hypoxia, and the ability of HBO2T rapidly to overcome this hypoxia. CO is eliminated rapidly via the lungs on application of HBO2T, with a half-life of about 21 min at 2.0 ATA versus 5.5 h breathing air and 71 min breathing oxygen at sea level. In practice, however, it seems unlikely that HBO2T can be delivered in time to prevent either acute hypoxic death or irreversible global cerebral hypoxic injury. If HBO2T is beneficial in CO poisoning, it must reduce the likelihood of persisting and/or delayed neurocognitive deficit through a mechanism other than the simple reversal of arterial hypoxia due to high levels of COHb. The difficulty in accurately assessing neurocognitive deficit has been one of the primary sources of controversy surrounding the clinical evidence in this area. To date there have been six randomized controlled trials of HBO2T for CO poisoning, although only four have been reported in full. While a Cochrane review suggested that overall there is insufficient evidence to confirm a beneficial effect of HBO2T on the chance of persisting neurocognitive deficit following poisoning (34% of patients treated with oxygen at 1 atmosphere vs 29%, of those treated with HBO2T; odds ratio [OR] 0.78; 95% CI 0.54–1.1), this may have more to do with poor reporting and inadequate follow-up than with evidence that HBO2T is not effective. The interpretation of the literature has much to do with how one defines neurocognitive deficit. In the most methodologically rigorous of these studies (Weaver et al.), a professionally administered battery of validated neuropsychological tests and a definition based on the deviation of individual subtest scores from the age-adjusted normal values was used; if the patient complained of memory, attention, or concentration difficulties, the required decrement was decreased. Using this approach, 6 weeks after poisoning, 46% of patients treated with normobaric oxygen alone had cognitive sequelae compared to 25% of those who received HBO2T (p = .007; number needed to treat [NNT] = 5; 95% CI 3–16). At 12 months, the difference remained significant (32% vs 18%; p = .04; NNT = 7; 95% CI 4–124) despite considerable loss to follow-up.
On this basis, HBO2T remains widely advocated for the routine treatment of patients with moderate to severe poisoning—in particular in those older than 35 years, presenting with a metabolic acidosis on arterial blood-gas analysis, exposed for lengthy periods, or with a history of unconsciousness. Conversely, many toxicologists remain unconvinced about the place of HBO2T in this situation and call for further well-designed studies.
DIVING MEDICINE
INTRODUCTION
Underwater diving is both a popular recreational activity and a means of employment in a range of tasks from underwater construction to military operations. It is a complex activity with unique hazards and medical complications arising mainly as a consequence of the dramatic changes in pressure associated with both descent and ascent through the water column. For every 10.13 m increase in depth of seawater, the ambient pressure (Pamb) increases by 101.3 kPa (1 atmosphere) so that a diver at 20 m depth is exposed to a Pamb of approximately 303.9 kPa (3 ATA), made up of 1 ATA due to atmospheric pressure and 2 ATA generated by the water column.
BREATHING EQUIPMENT
Most diving is undertaken using a self-contained underwater breathing apparatus (scuba) consisting of one or more cylinders of compressed gas connected to a pressure-reducing regulator and a demand valve activated by inspiratory effort. Some divers use “rebreathers,” which are scuba devices that are closed or semiclosed circle systems with a carbon dioxide scrubber and a system designed to maintain a safe inspired PO2. Exhaled gas is recycled, and gas consumption is limited to little more than the oxygen metabolized by the diver. Rebreathers are therefore popular for deep dives where expensive helium is included in the respired mix (see below). Occupational divers frequently use “surface supply” equipment where gas, along with other utilities such as communications and power, is supplied via an umbilical from the surface.
All these systems must supply gas to the diver at the Pamb of the surrounding water or inspiration would be impossible against the surrounding water pressure. For most recreational diving, the respired gas is air. Pure oxygen is rarely used because oxygen may provoke seizures above an inspired PO2 of 162 kPa (1.6 ATA) in aquatic environments, limiting the practical safe depth to 6 m. This is a conspicuously lower PO2 than routinely used for hyperbaric therapy, reflecting a higher risk of both seizures and pulmonary toxicity during immersion. For the same reason, very deep diving requires the use of oxygen fractions lower than in air (FO2 0.21). This is because breathing air at 66 m means inspiring 1.6 ATA of oxygen, the maximum allowable pressure. To dive any deeper, breathing gases must contain less oxygen than air. Deep-diving gases often include helium instead of nitrogen to reduce both the narcotic effect and high gas density that result from breathing nitrogen at high pressures.
SUITABILITY FOR DIVING
The most common reason for physician consultation in relation to diving is for the evaluation of suitability for diver training or after a health event. Occupational diver candidates are usually compelled to see doctors with specialist training in the field, both at entry to the industry and periodically thereafter, and their medical evaluations are usually conducted according to legally mandated standards. In contrast, in most jurisdictions prospective recreational diver candidates simply complete a self-assessment medical questionnaire prior to diver training. If there are no positive responses, the candidate proceeds directly to training, but positive responses mandate the candidate see a doctor for evaluation of the identified medical issue. Prospective divers will often present to their family medicine practitioner for this purpose. In the modern era, such consultations have evolved from a simple proscriptive exercise of excluding those with potential contraindications to a more “risk analysis” approach in which each case is evaluated on its own merits. Such analyses require integration of diving physiology, the impact of associated medical problems, and a detailed knowledge of the specific medical condition of the candidate. A detailed discussion of the subject is beyond the scope of this chapter, but a few important principles are outlined below.
There are three primary questions that should be answered: (1) Could the underlying condition be exacerbated by diving? (2) Could the condition make a diving medical problem more likely? (3) Could the condition prevent the diver from meeting the functional requirements of diving? As examples, epilepsy is usually considered a contraindication because there are epileptogenic stimuli encountered in diving that could make a seizure more likely (such as thermal stress and exercise). Active asthma is a relative contraindication because it could predispose to pulmonary barotrauma (see below), and untreated ischemic heart disease is a contraindication because it could prevent a diver from exercising sufficiently to get out of a difficult situation such as being caught in a current. It can be a complex matter to recognize the relevant interactions between diving and medical conditions and to determine the impact on suitability for diving. Physicians interested in regularly conducting such evaluations should obtain relevant training. Short courses providing relevant training are offered by specialist groups in most countries.
BAROTRAUMA
The problem of middle-ear barotrauma (MEBT) with diving is similar to the problem that may occur during descent from altitude in an airplane, but difficulties with equalizing pressure in the middle ear are exaggerated underwater by both the rapidity and magnitude of pressure change as a diver descends or ascends. Failure to periodically insufflate the middle-ear spaces via the eustachian tubes during descent results in increasing pain. As the Pamb increases, the tympanic membrane (TM) may be bruised or even ruptured as it is pushed inward. Negative pressure in the middle ear results in engorgement of blood vessels in the mucous membranes and leads to effusion or bleeding, which can be associated with a conductive hearing loss. MEBT is much less common during ascent because expanding gas in the middle-ear space tends to open the eustachian tube easily and “automatically.” Barotrauma may also affect the respiratory sinuses, although the sinus ostia are usually widely patent and allow automatic pressure equalization without the need for specific maneuvers. If equalization fails, pain usually results in termination of the dive. Difficulty with equalizing ears or sinuses may respond to oral or nasal decongestants.
Much less commonly the inner ear may suffer barotrauma (IEBT). Several explanations have been proposed, of which the most favored holds that forceful attempts to insufflate the middle-ear space by Valsalva maneuvers during descent cause sudden transmission of pressure to the perilymph via the cochlear aqueduct and outward rupture of the round window already under tension because of negative middle ear pressure. The clinician should be alerted to possible IEBT after diving by a sensorineural hearing loss or true vertigo (which is often accompanied by nausea, vomiting, nystagmus, and ataxia). These manifestations can also occur in vestibulocochlear DCS (see below) but should never be attributed to MEBT. Immediate review by an expert diving physician is recommended, and urgent referral to an otologist will often follow.
The lungs are also vulnerable to barotrauma but are at most risk during ascent. If expanding gas becomes trapped in the lungs as Pamb falls, this may rupture alveoli and associated vascular tissue. Gas trapping may occur if divers intentionally or involuntarily hold their breath during ascent or if there are bullae. The extent to which asthma predisposes to pulmonary barotrauma is debated, but the presence of active bronchoconstriction must increase risk. For this reason, asthmatics who regularly require bronchodilator medications or whose airways are sensitive to exercise or cold air are usually discouraged from diving. While possible consequences of pulmonary barotrauma include pneumothorax and mediastinal emphysema, the most feared is the introduction of gas into the pulmonary veins leading to cerebral arterial gas embolism (CAGE). Manifestations of CAGE include loss of consciousness, confusion, hemiplegia, visual disturbances, and speech difficulties, appearing immediately or within minutes after surfacing. The management is the same as for DCS described below. It is notable that the natural history of CAGE often includes substantial or complete resolution of symptoms early after the event. This is probably the clinical correlate of bubble involution and redistribution with consequent restoration of flow. Patients exhibiting such remissions should still be reviewed at specialist diving medical centers because secondary deterioration or reembolization can occur. Unsurprisingly, these events can be misdiagnosed as typical strokes or transient ischemic attacks (TIAs) (Chap. 455) when patients are seen by those unfamiliar with diving medicine. All patients presenting with neurologic symptoms after diving should have their symptoms discussed with a specialist in diving medicine and be considered for recompression therapy.
DECOMPRESSION SICKNESS
DCS is caused by the formation of bubbles from dissolved inert gas (usually nitrogen) during or after ascent (decompression) from a compressed gas dive. Bubble formation is also possible following decompression for extravehicular activity during space flight and with ascent to altitude in unpressurized aircraft. DCS in the latter scenarios is probably rare in comparison with diving, where the incidence is approximately 1:10,000 recreational dives.
Breathing at elevated Pamb results in increased uptake of inert gas into blood and then into tissues. The rate at which tissue inert gas equilibrates with the inspired inert gas pressure is proportional to tissue blood flow and the blood-tissue partition coefficient for the gas. Similar factors dictate the kinetics of inert gas washout during ascent. If the rate of gas washout from tissues does not match the rate of decline in Pamb, then the sum of dissolved gas pressures in the tissue will exceed Pamb, a condition referred to as “supersaturation.” This is the prerequisite for bubbles to form during decompression, although other less well-understood factors are also involved. Deeper and longer dives result in greater inert gas absorption and greater likelihood of tissue supersaturation during ascent. Divers control their ascent for a given depth and time exposure using algorithms that often include periods where ascent is halted for a prescribed period at different depths to allow time for gas washout (“decompression stops”). Although a breach of these protocols increases the risk of DCS, adherence does not guarantee against it. DCS should be considered in any diver manifesting symptoms not readily explained by an alternative mechanism.
Bubbles may form within tissues themselves, where they cause symptoms by mechanical distraction of pain-sensitive or functionally important structures. They also appear in the venous circulation as blood passes through supersaturated tissues. Some venous bubbles are tolerated without symptoms and are filtered from the circulation in the pulmonary capillaries. However, in sufficiently large numbers, these bubbles are capable of inciting inflammatory and coagulation cascades, damaging endothelium, activating formed elements of blood such as platelets, and causing symptomatic pulmonary vascular obstruction. Moreover, if there is a right-to-left shunt such as through a patent foramen ovale (PFO) or an intrapulmonary shunt, then venous bubbles may enter the arterial circulation (25% of adults have a probe-patent PFO). The risk of cerebral, spinal cord, inner ear, and skin manifestations appears higher in the presence of significant shunts, suggesting that these “arterialized” venous bubbles can cause harm, perhaps by disrupting flow in the microcirculation of target organs. Circulating endothelial microparticles, which are elevated in number and size after diving, are currently under investigation as indicators of decompression stress or possibly as injurious agents in their own right. How they arise, and their role in DCS, remain unclear.
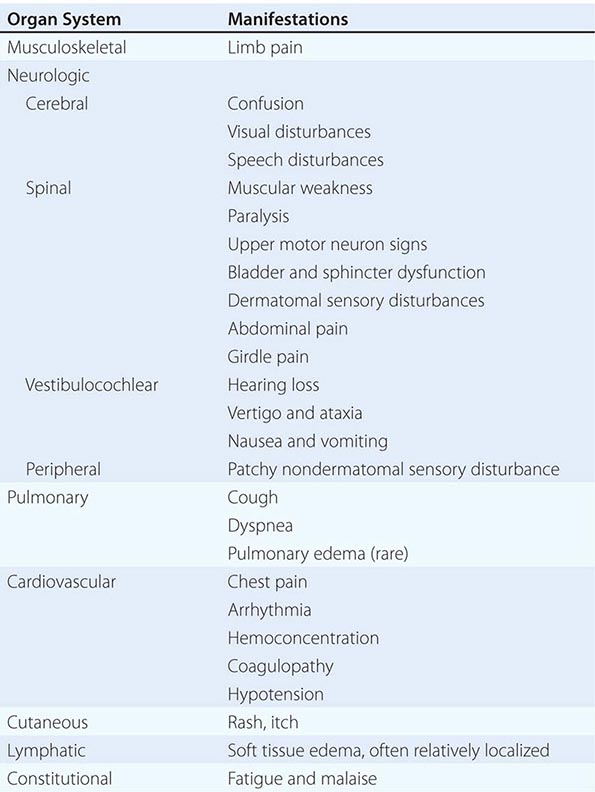
Table 477e-3 lists manifestations of DCS grouped according to organ system. The majority of cases present with mild symptoms, including musculoskeletal pain, fatigue, and minor neurologic manifestations such as patchy paresthesias. Serious presentations are much less common. Pulmonary and cardiovascular manifestations can be life-threatening, and spinal cord involvement frequently results in permanent disability. Latency is variable. Serious DCS usually manifests within minutes of surfacing, but mild symptoms may not appear for several hours. Symptoms arising more than 24 h after diving are very unlikely to be DCS. The presentation may be confusing and nonspecific, and there are as yet no useful diagnostic investigations. Diagnosis is based on integration of findings from examination of the dive profile, the nature and temporal relationship of symptoms, and the clinical examination. Some DCS presentations may be difficult to separate from CAGE following pulmonary barotrauma, but from a clinical perspective the distinction is unimportant because the first aid and definitive management of both conditions are the same.
MANIFESTATIONS OF DECOMPRESSION SICKNESS |