Human Immunodeficiency Virus Infection
KEY CONCEPTS
![]() Infection with human immunodeficiency virus (HIV) occurs through three primary modes: sexual, parenteral, and perinatal. Sexual intercourse, primarily receptive anal and vaginal intercourse, is the most common method for transmission.
Infection with human immunodeficiency virus (HIV) occurs through three primary modes: sexual, parenteral, and perinatal. Sexual intercourse, primarily receptive anal and vaginal intercourse, is the most common method for transmission.
![]() HIV infects cells expressing cluster of differentiation 4 (CD4) receptors, such as T-helper lymphocytes, monocytes, macrophages, dendritic cells, and brain microglia. Infection occurs via an interaction between glycoprotein 160 (gp160) on HIV with CD4 (primary interaction) and chemokine coreceptors (secondary interactions) present on the surfaces of these cells.
HIV infects cells expressing cluster of differentiation 4 (CD4) receptors, such as T-helper lymphocytes, monocytes, macrophages, dendritic cells, and brain microglia. Infection occurs via an interaction between glycoprotein 160 (gp160) on HIV with CD4 (primary interaction) and chemokine coreceptors (secondary interactions) present on the surfaces of these cells.
![]() The hallmark of untreated HIV infection is profound CD4 T-lymphocyte depletion and severe immunosuppression that puts patients at significant risk for infectious diseases caused by opportunistic pathogens. Opportunistic infections (OIs) in settings without access to antiretroviral drugs are the chief cause of morbidity and mortality associated with HIV infection.
The hallmark of untreated HIV infection is profound CD4 T-lymphocyte depletion and severe immunosuppression that puts patients at significant risk for infectious diseases caused by opportunistic pathogens. Opportunistic infections (OIs) in settings without access to antiretroviral drugs are the chief cause of morbidity and mortality associated with HIV infection.
![]() The current goal of ART is to achieve maximal and durable suppression of HIV replication, taken to be a level of HIV-RNA in plasma (viral load) less than the lower limit of quantitation. Another equally important outcome is an increase in CD4 lymphocytes because this closely correlates with the risk for developing OIs.
The current goal of ART is to achieve maximal and durable suppression of HIV replication, taken to be a level of HIV-RNA in plasma (viral load) less than the lower limit of quantitation. Another equally important outcome is an increase in CD4 lymphocytes because this closely correlates with the risk for developing OIs.
![]() General principles for the management of OIs include preventing or reversing immunosuppression with antiretroviral therapy (ART), preventing exposure to pathogens, vaccination, prospective immunologic monitoring, primary chemoprophylaxis, treatment of acute episodes, secondary chemoprophylaxis, and discontinuation of such prophylaxes following ART and subsequent immune recovery.
General principles for the management of OIs include preventing or reversing immunosuppression with antiretroviral therapy (ART), preventing exposure to pathogens, vaccination, prospective immunologic monitoring, primary chemoprophylaxis, treatment of acute episodes, secondary chemoprophylaxis, and discontinuation of such prophylaxes following ART and subsequent immune recovery.
![]() Clinical use of antiretroviral agents is complicated by drug–drug interactions. Some interactions are beneficial and used purposely; others may be harmful, leading to dangerously elevated or inadequate drug concentrations. For these reasons, clinicians involved in the pharmacotherapy of HIV infection must exercise constant vigilance and maintain a current knowledge of drug interactions.
Clinical use of antiretroviral agents is complicated by drug–drug interactions. Some interactions are beneficial and used purposely; others may be harmful, leading to dangerously elevated or inadequate drug concentrations. For these reasons, clinicians involved in the pharmacotherapy of HIV infection must exercise constant vigilance and maintain a current knowledge of drug interactions.
![]() Current recommendations for the initial treatment of HIV advocate a minimum of three active antiretroviral agents from at least two drug classes. The typical regimen consists of two nucleoside/nucleotide analogs with either a protease inhibitor (PI; pharmacokinetically enhanced by coadministration with a CYP3A inhibitor), a nonnucleoside reverse transcriptase inhibitor, or an integrase strand transfer inhibitor (InSTI).
Current recommendations for the initial treatment of HIV advocate a minimum of three active antiretroviral agents from at least two drug classes. The typical regimen consists of two nucleoside/nucleotide analogs with either a protease inhibitor (PI; pharmacokinetically enhanced by coadministration with a CYP3A inhibitor), a nonnucleoside reverse transcriptase inhibitor, or an integrase strand transfer inhibitor (InSTI).
![]() Inadequate suppression of viral replication allows HIV to select for antiretroviral-resistant HIV variants, a major factor limiting the ability of antiretroviral drugs to inhibit virus replication. Current recommendations for treating drug-resistant HIV include choosing at least two drugs (preferably three) to which the patient’s virus is susceptible. Susceptibility can be assessed using either (virtual) genotypic or phenotypic resistance testing.
Inadequate suppression of viral replication allows HIV to select for antiretroviral-resistant HIV variants, a major factor limiting the ability of antiretroviral drugs to inhibit virus replication. Current recommendations for treating drug-resistant HIV include choosing at least two drugs (preferably three) to which the patient’s virus is susceptible. Susceptibility can be assessed using either (virtual) genotypic or phenotypic resistance testing.
![]() The reduction of viral load with ART lowers the risk of transmission to others. Additionally, prophylaxis with antiretroviral agents in at-risk persons lowers HIV acquisition risk.
The reduction of viral load with ART lowers the risk of transmission to others. Additionally, prophylaxis with antiretroviral agents in at-risk persons lowers HIV acquisition risk.
![]() The longer life span conferred by antiretroviral treatment has given rise to other medical issues. First, a wide spectrum of complications associated with older age have become common, some of which are adverse effects from antiretroviral drugs. Second, hepatitis C virus (HCV) coinfection is an important cause of morbidity and mortality. Medical management of these contemporary HIV complications is constantly evolving.
The longer life span conferred by antiretroviral treatment has given rise to other medical issues. First, a wide spectrum of complications associated with older age have become common, some of which are adverse effects from antiretroviral drugs. Second, hepatitis C virus (HCV) coinfection is an important cause of morbidity and mortality. Medical management of these contemporary HIV complications is constantly evolving.
Acquired immunodeficiency syndrome (AIDS) was first recognized in a cohort of young, previously healthy homosexual men with new-onset profound immunologic deficits, Pneumocystis carinii (now P. jirovecii) pneumonia (PCP), and/or Kaposi’s sarcoma. A retrovirus, human immunodeficiency virus type 1 (HIV-1), is the major cause of AIDS. A second retrovirus, HIV-2, also is recognized to cause AIDS, although it is less virulent, transmissible, and prevalent than HIV-1. These retroviruses are transmitted primarily by sexual contact and by contact with infected blood or blood products. Several risk behaviors for the acquisition of HIV infection have been identified in the United States, most notably the practice of anorectal intercourse and the sharing of blood-contaminated needles by injection-drug users. In many resource-limited countries, the majority of HIV transmission occurs via heterosexual intercourse and from childbearing women to their offspring. Initially, the medical management of HIV consisted of repeated treatments for opportunistic infections (OIs) and eventual palliative care. In the mid-1990s, a new era in the pharmacotherapy for HIV, known as combination antiretroviral therapy (ART), was born. ART consists of combinations of antiretroviral agents with different mechanisms of action that potently and durably suppress HIV replication, delay the onset of AIDS, reverse HIV-associated immunologic deficits, reduce HIV transmissions, and significantly prolong survival. Modern antiretroviral drugs and ART regimens have improved upon tolerability and efficacy. Unfortunately, therapeutic challenges remain in the ART era and include the need for continuous adherence to medication and care, drug–drug interactions, drug-resistant HIV, acute and long-term drug toxicities, and other complications associated with a prolonged life span. Progress has been made in the treatment access for this disease, but large numbers of HIV-infected persons remain outside of care. Antiretroviral drugs can prevent HIV acquisition in persons exposed to HIV, but they cannot cure established HIV infection, and no viable vaccine is available.
EPIDEMIOLOGY
The epidemiologic characteristics of HIV infection differ according to geographic region and depend upon the mode of transmission, governmental prevention efforts and resources, and cultural factors.1,2
![]() Infection with HIV occurs through three primary modes: sexual, parenteral, and perinatal. Sexual intercourse, primarily anal and vaginal intercourse, is the most common method for transmission. The probability of HIV transmission depends upon the type of sexual exposure. The highest risk appears to be from receptive anorectal intercourse at about 0.5% to 3% per sexual act.3 Transmission risk is lower for receptive vaginal or oral intercourse and each is lower for insertive versus receptive sex acts.4 Condom use reduces risk of transmission by approximately 20-fold.4 Other factors that affect the probability of infection include the stage of HIV disease and viral load in the index partner. For example, transmission is higher when the index partner has early or late HIV compared with asymptomatic HIV, as these disease stages are associated with higher viral loads.5 Individuals with genital ulcers or sexually transmitted diseases are at greater risk for contracting HIV. HIV incidence and prevalence are lower in cultures that advocate male circumcision, which is estimated to reduce risk of male acquisition of HIV during heterosexual intercourse by 60%.2,5 However, male circumcision may not have the same protective effects for receptive anal intercourse or for an uninfected partner.6 Casual contact with patients with AIDS or HIV infection is not a significant risk factor for HIV transmission.2
Infection with HIV occurs through three primary modes: sexual, parenteral, and perinatal. Sexual intercourse, primarily anal and vaginal intercourse, is the most common method for transmission. The probability of HIV transmission depends upon the type of sexual exposure. The highest risk appears to be from receptive anorectal intercourse at about 0.5% to 3% per sexual act.3 Transmission risk is lower for receptive vaginal or oral intercourse and each is lower for insertive versus receptive sex acts.4 Condom use reduces risk of transmission by approximately 20-fold.4 Other factors that affect the probability of infection include the stage of HIV disease and viral load in the index partner. For example, transmission is higher when the index partner has early or late HIV compared with asymptomatic HIV, as these disease stages are associated with higher viral loads.5 Individuals with genital ulcers or sexually transmitted diseases are at greater risk for contracting HIV. HIV incidence and prevalence are lower in cultures that advocate male circumcision, which is estimated to reduce risk of male acquisition of HIV during heterosexual intercourse by 60%.2,5 However, male circumcision may not have the same protective effects for receptive anal intercourse or for an uninfected partner.6 Casual contact with patients with AIDS or HIV infection is not a significant risk factor for HIV transmission.2
Prevention of sexual transmission has focused primarily on education that encourages abstinence (especially for adolescents), use of condoms, and reduction of high-risk behavior (anal intercourse or promiscuity with partners of unknown HIV status).1 Combination ART dramatically lowers viral replication and infectiousness, significantly reducing the risk of transmission to others.7 Chemoprophylaxis with antiretroviral drugs is also effective at preventing HIV acquisition.8–10 A combined approach has been advocated for optimal prevention. Prevention strategies under investigation include HIV vaccines and topical vaginal/rectal microbicides.11,12
Parenteral transmission of HIV broadly encompasses infections due to infected blood exposure from needle sticks, IV injection with used needles, receipt of blood products, and organ transplants. Use of contaminated needles or other injection-related paraphernalia by drug abusers has been the main cause of parenteral transmissions. The risk of HIV transmission from sharing needles is approximately 0.67% per episode.3 Prevention strategies include stopping drug abuse, obtaining needles from credible sources (e.g., pharmacies), never reusing any paraphernalia, using sterile procedures in all injecting activities, and safely disposing of used paraphernalia.4
Before widespread screening, HIV was readily transmitted in blood products.3 However, blood and tissue products in the healthcare system are now rigorously screened for HIV. The estimated risk for receiving tainted blood or blood products in the United States is approximately 1:2,000,000 and that for receiving a tainted tissue transplant is 1:55,000.13,14 Healthcare workers have a small but definite occupational risk of contracting HIV through accidental injury. Most cases of occupationally acquired HIV have been the result of a percutaneous needle stick injury, which carries an estimated 0.3% risk of transmitting HIV.15 Mucocutaneous exposures (e.g., tainted blood splash in eyes, mouth, nose) carries a transmission risk of approximately 0.09%.15 Significant risk factors for seroconversion with a needle stick include deep injury, injury with a device visibly contaminated with blood, and advanced HIV disease in the index patient (high viral load). The risk of transmission from an HIV-infected healthcare worker to a patient is extremely remote. Comprehensive medical guidelines, including antiretroviral drug prophylaxis, have been developed to minimize the hazard of HIV transmission for healthcare workers and for persons exposed by rape or other means.3,15
Perinatal infection, or vertical transmission, is the most common cause of pediatric HIV infection.16 Most infections occur during or near to the time of birth, although a fraction can occur in utero. The risk of mother-to-child transmission is approximately 25% in the absence of ART. Factors that increase the likelihood of vertical transmission include prolonged rupture of membranes, chorioamnionitis, genital infection during pregnancy, preterm delivery, vaginal delivery, birth weight less than 2.5 kg, illicit drug use during pregnancy, and high maternal viral load. Breast-feeding also can transmit HIV. The estimated frequency of breast milk transmission is approximately 4% to 16%, with the majority of infections developing within the first 6 months.17 High levels of virus in breast milk and in the mother are associated with higher risk of transmission.17 Formula feeding prevents breast milk transmission of HIV but may not improve mortality from other causes early in life in some settings.18 Whenever formula feeding is acceptable, feasible, affordable, sustainable, and safe, HIV-infected mothers are recommended not to breast-feed. A separate and comprehensive set of medical guidelines including antiretroviral drug prophylaxis have been developed to minimize the hazard of mother-to-child HIV transmission.16
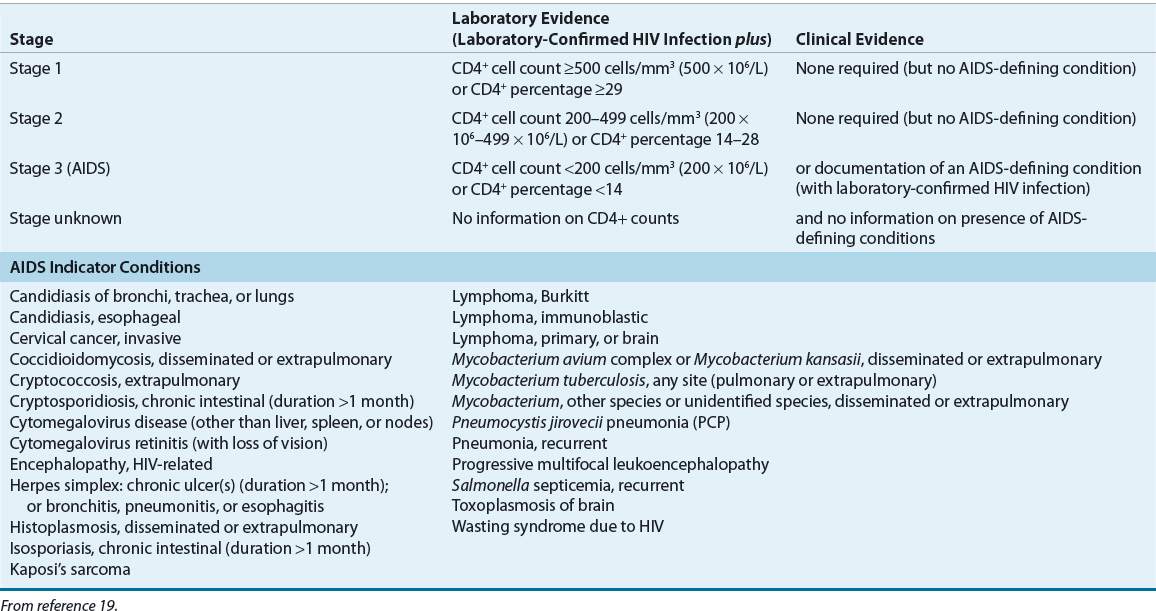
Persons with HIV infection are broadly categorized as those living with HIV and those with an AIDS diagnosis. An AIDS diagnosis is made when the presence of HIV is laboratory-confirmed and the cluster of differentiation 4 (CD4; T-helper cell) count drops below 200 cells/mm3 (200 × 106/L) or after an AIDS indicator condition is diagnosed.19 Further distinctions regarding the stage of HIV and AIDS are given in the Revised Centers for Disease Control and Prevention (CDC) surveillance case definition (Table 103-1).19 In the United States, new HIV/AIDS cases are reported by healthcare providers to a public health department.20 The cumulative number of reported HIV/AIDS diagnoses in the United States is approximately 1.7 million; more than 550,000 persons have already died.21 The estimated prevalence of HIV infections including AIDS cases in the United States is about 1.2 million individuals. Each year the CDC estimates that 55,000 new cases of HIV infection occur in the United States.21,22 Approximately 20% of persons with HIV are unaware of their infection and approximately 50% of those who are aware of their infection are retained in care.22,23 Therefore, the majority of HIV-infected persons (∼60%) are not receiving ART regularly, which contributes to the ongoing transmission of HIV infection.23
TABLE 103-1 Surveillance Case Definition for HIV Infection among Adults and Adolescents (≥13 years)—United States, 2008
The epidemic in the United States initially was established in white men who have sex with men (MSM), and the prevalence of HIV in this population still is high.22 New trends in transmission include more cases in women (currently ∼25%) and African Americans and Hispanics, a proportion of whom are not well linked to appropriate prevention, care, and treatment services.21 Approximately half of new cases occur in African Americans (who make up only 12% of the general population), about one third in Caucasians, and less than one fourth in Hispanics.20 The main risk factor for transmission in women is heterosexual intercourse (∼80% of cases) and injection-drug use (∼20% of cases). For men the main risks are MSM (∼65%), heterosexual sex (∼15%), and injection-drug use (∼15%).24
The estimated number of individuals living with HIV/AIDS worldwide has stabilized at approximately 34 million persons. The new infection rate is declining.25 Approximately 2.7 million were infected in 2010, including 390,000 children, down from approximately 3.3 million new infections in 1998.25 About 1.8 million people succumbed to AIDS in 2010. Globally, the highest concentration of HIV/AIDS cases is in sub-Saharan Africa, where approximately 23 million people are infected. However, new infections rates in many sub-Saharan African countries have declined by approximately 25% since 1997.25 Heterosexual transmission is the most common mode of transmission in sub-Saharan Africa and worldwide (85% of cases). Women in sub-Saharan Africa and resource-limited countries are at disproportionately high risk for acquiring HIV because of biological and cultural factors that foster HIV transmission, such as limited ability to refuse sex.1,25 Other important epidemiologic features of the HIV epidemic include growing prevalence in eastern Europe and central Asia (e.g., Russian Federation and Ukraine).25 Injection-drug use is fueling these epidemics.
ETIOLOGY
HIV is an enveloped single-stranded RNA virus and a member of the Lentivirinae (lenti, meaning “slow”) subfamily of retroviruses. Lentiviruses are characterized by their indolent infectious cycle. There are two related but distinct types of HIV: HIV-1 and HIV-2. HIV-2, found mostly in western Africa, consists of seven phylogenetic lineages designated as subtypes (clades) A through G. HIV-1 also can be categorized based on phylogeny.26 Three groups of HIV-1 are recognized: M (main or major), N (non-M, non-O), and O (outlier). A new HIV-1 virus was classified as group P (pending the identification of further cases).27 The nine subtypes of HIV-1 group M are identified as A through D, F through H, and J and K. Mixtures of subtypes are referred to as circulating recombinant forms (CRFs). Group M, subtype B, is primarily responsible for the epidemic in North America and western Europe.26
The accumulated evidence suggests that HIV in humans was the result of a cross-species transmission (zoonosis) from primates infected with simian immunodeficiency virus (SIV). Phylogenetic and geographic relationships suggest that HIV-2 arose from SIV that infects sooty mangabeys and HIV-1 group M and N arose from SIVcpz, a virus that infects chimpanzees (Pan troglodytes troglodytes). Groups O and P may have arisen from a SIV variant that infects wild gorillas. Cultural practices, such as preparation and eating of bush meat or keeping animals as pets, may have allowed the virus to jump from primates to humans. The earliest known human infection with HIV has been traced to central Africa in 1959, but cross-species transmissions probably date back to the early 1900s.28 Modern transportation, promiscuity, and drug abuse have caused the rapid spread of the virus within the United States and throughout the world.1,28 This chapter focuses on HIV-1 group M, which is the predominant strain likely to be encountered in the western world.
DETECTION OF HIV AND SURROGATE MARKERS OF DISEASE PROGRESSION
The preferred method for diagnosing HIV-1 infection is an enzyme-linked immunosorbent assay (ELISA), which detects antibodies against HIV-1.24 ELISA is both highly sensitive (>99%) and highly specific (>99%), but rare false-positive results can occur in multiparous women; recent recipients of hepatitis B, HIV, influenza, or rabies vaccine; patients with multiple blood transfusions, liver disease, and renal failure; or those undergoing chronic hemodialysis. False-negative results may occur and most commonly are attributed to new infection where antibody production is not yet adequate. An HIV-RNA test can detect viremia approximately 2 weeks prior to antibody production.29 The minimum time to develop antibodies is 3 to 4 weeks from initial exposure, with greater than 95% of individuals developing antibodies after 6 months. Convenient methods for obtaining an ELISA sample from blood or saliva have been developed, including a rapid (20 to 40 minutes) turnaround oral test marketed as a home kit.
Positive ELISA results are repeated in duplicate, and if one or both tests are reactive, a confirmatory test is performed for final diagnosis. Western blot is the most commonly used confirmatory test, although an indirect immunofluorescence assay is available. A reactive ELISA test and a positive confirmatory test indicate an established HIV infection. If the confirmatory test is indeterminate, the individual should be retested 4 weeks later.24
HIV testing is recommended when HIV infection is suspected because of symptoms and/or high-risk behavior. Additionally, the CDC now recommends routine HIV screening in all healthcare settings in persons 13 to 64 years, a new policy called “opt-out” testing.24 The policy states that consent for medical care will imply consent for HIV testing; however, the person must be informed of the test and can opt out of taking it. Because states may have different HIV consent laws, the local requirements for HIV testing should be consulted. The rationale for the opt-out strategy is to diagnose those who unknowingly carry HIV so as to improve their prognosis and reduce further transmission.
Once diagnosed, HIV disease is monitored primarily by two surrogate biomarkers, viral load and CD4 cell count.30 The viral load test quantifies the degree of viremia by measuring the number of copies of viral RNA (HIV RNA) in the plasma. Methods for determining HIV RNA include reverse-transcription polymerase chain reaction (RT-PCR), branched-chain DNA, transcription-mediated amplification, and nucleic acid sequence-based assay. RT-PCR is used more widely than the other techniques. Irrespective of the method used, viral load is reported as the number of viral RNA copies per milliliter of plasma. Each assay has its own lower limit of sensitivity to viral subtypes, and results can vary from one assay method to the other; therefore, it is recommended that the same assay method be used consistently within patients. Reductions in viral load often are reported in base 10 logarithm. For example, if a patient presents initially with a viral load of 100,000 copies/mL (105 copies/mL or 108 copies/L) and subsequently has a viral load of 10,000 copies/mL (104 copies/mL or 107 copies/L), the decrease in viral load is 1 log10. Given that HIV RNA varies within patients, a clinical response is generally considered when the decline in viral load is more than 0.5 log10.30 Viral load is a major prognostic factor for monitoring disease progression and the effects of treatment.
Because HIV attacks and leads to the destruction of cells bearing the CD4 receptor, the number of CD4 lymphocytes (T-helper cells) in the blood is a critical surrogate marker of disease progression. The normal adult CD4 lymphocyte count ranges from 500 to 1,600 cells/mm3, or 40% to 70% of total lymphocytes. CD4 counts in children are age dependent, with younger children having higher CD4 counts. The hallmark of HIV disease is depletion of CD4 cells and the associated development of OIs and malignancies.
PATHOGENESIS
![]() Understanding the life cycle of HIV (Fig. 103-1) is necessary because the current strategies used for treatment of HIV target various points in this cycle. Once HIV enters the human body, the outer glycoprotein (gp160) on its surface, which is composed of two subunits (gp120 and gp41), has affinity for CD4 receptors, proteins present on the surface of T-helper lymphocytes, monocytes, macrophages, dendritic cells, and brain microglia.31 The gp120 subunit is responsible for CD4 binding. Once initial binding occurs, the intimate association of HIV with the cell is enhanced by further binding to chemokine coreceptors. The two major chemokine receptors used by HIV are Chemokine (C–C motif) receptor 5 (CCR5) and chemokine (C-X-C motif) receptor 4 (CXCR4). HIV isolates may contain a mixture of viruses that target one or the other of these coreceptors, and some viral strains may be dual-tropic (i.e., can use both coreceptors). The HIV strain that preferentially uses CCR5, R5 viruses, is macrophage-tropic and typically implicated in most cases of sexually transmitted HIV. Individuals with a common 32-base-pair deletion in the CCR5 gene are protected from progression of HIV disease, and those who are homozygous for the 32-base-pair deletion have a degree of resistance to acquisition of HIV-1.32,33 The HIV strain that targets CXCR4, designated X4 virus, is T-cell–tropic and often is predominant in the later stage of disease. Other chemokine coreceptors and galactosyl ceramide may also serve as a binding site for HIV. CD4 and coreceptor attachment of HIV to the cell promotes membrane fusion, which is mediated by gp41, and finally internalization of the viral genetic material and enzymes necessary for replication.
Understanding the life cycle of HIV (Fig. 103-1) is necessary because the current strategies used for treatment of HIV target various points in this cycle. Once HIV enters the human body, the outer glycoprotein (gp160) on its surface, which is composed of two subunits (gp120 and gp41), has affinity for CD4 receptors, proteins present on the surface of T-helper lymphocytes, monocytes, macrophages, dendritic cells, and brain microglia.31 The gp120 subunit is responsible for CD4 binding. Once initial binding occurs, the intimate association of HIV with the cell is enhanced by further binding to chemokine coreceptors. The two major chemokine receptors used by HIV are Chemokine (C–C motif) receptor 5 (CCR5) and chemokine (C-X-C motif) receptor 4 (CXCR4). HIV isolates may contain a mixture of viruses that target one or the other of these coreceptors, and some viral strains may be dual-tropic (i.e., can use both coreceptors). The HIV strain that preferentially uses CCR5, R5 viruses, is macrophage-tropic and typically implicated in most cases of sexually transmitted HIV. Individuals with a common 32-base-pair deletion in the CCR5 gene are protected from progression of HIV disease, and those who are homozygous for the 32-base-pair deletion have a degree of resistance to acquisition of HIV-1.32,33 The HIV strain that targets CXCR4, designated X4 virus, is T-cell–tropic and often is predominant in the later stage of disease. Other chemokine coreceptors and galactosyl ceramide may also serve as a binding site for HIV. CD4 and coreceptor attachment of HIV to the cell promotes membrane fusion, which is mediated by gp41, and finally internalization of the viral genetic material and enzymes necessary for replication.
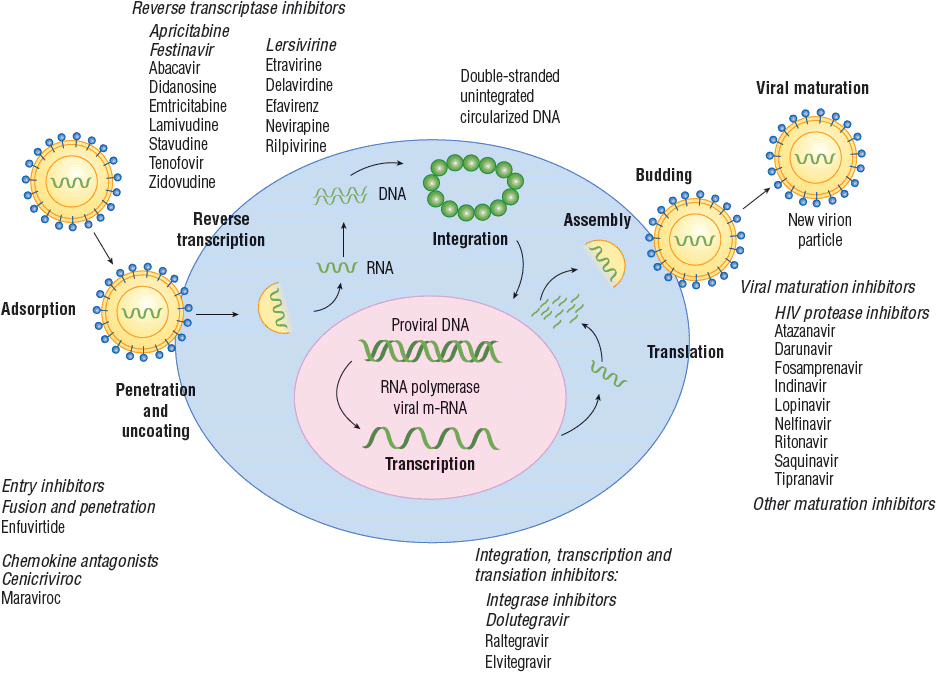
FIGURE 103-1 Life cycle of human immunodeficiency virus with potential targets where replication may be interrupted. Italicized compounds were in development at the time of this writing. (Reprinted with permission, Courtney V. Fletcher, 2012.)
After internalization, the viral protein shell surrounding the nucleic acid (capsid) is uncoated in preparation for replication.31 The genetic material of HIV is positive-sense single-stranded RNA; the virus must transcribe this RNA into DNA (transcription normally occurs from DNA to RNA; HIV works backward, hence the name retrovirus). To do so, HIV is equipped with the unique enzyme RNA-dependent DNA polymerase (reverse transcriptase). HIV reverse transcriptase first synthesizes a complementary strand of DNA using the viral RNA as a template. The RNA portion of this DNA–RNA hybrid is then partially removed by ribonuclease H (RNase H), allowing HIV reverse transcriptase to complete the synthesis of a double-stranded DNA molecule. The fidelity of HIV reverse transcriptase is poor, and many mistakes are made during the process. These errors in the final DNA product contribute to the rapid mutation of the virus, which enables the virus to evade the immune response (thus complicating vaccine development), and promotes the evolution of drug resistance during partially suppressive therapy. Following reverse transcription, the final double-stranded DNA product migrates into the nucleus and is integrated into the host cell chromosome by integrase, another enzyme unique to HIV.
The integration of HIV into the host chromosome is troublesome. Most notably, HIV can establish a persistent, latent infection, particularly in long-lived cells of the immune system such as memory T lymphocytes. The virus is effectively hidden in these cells, and this characteristic has greatly inhibited the ability to cure HIV infection. Second, random integration of HIV may cause cellular abnormalities and induce apoptosis.
After integration, HIV preferentially replicates in activated cells. Activation by antigens, cytokines, or other factors stimulates the cell to produce nuclear factor kappa B (NF-κ B), an enhancer-binding protein. NF-κ B normally regulates the expression of T-lymphocyte genes involved in growth but also can inadvertently activate replication of HIV. HIV encodes six regulatory and accessory proteins: Tat, Nef, Rev, Vpu, Vif, and Vpr, which enhance replication and inhibit innate immunity. For example, the Tat protein is a potent amplifier of HIV gene expression; it binds to a specific RNA sequence of HIV that initiates and stabilizes transcription elongation. Vif is a viral protein that binds human ABOBEC 3G, a cytidine deaminase that converts viral RNA cytosine to uracil and thereby provides innate cellular immunity.34 Vpu inhibits tetherin, a human cellular membrane protein that prevents diffusion of virus particles after budding from infected cells, thereby allowing HIV to detach from the infected cell.35 Assembly of new virion particles occurs in a stepwise manner beginning with the coalescence of HIV proteins beneath the host cell lipid bilayer. The nucleocapsid subsequently is formed with viral single-stranded RNA and other components packaged inside. Once packaged, the virion then buds through the plasma membrane, acquiring the characteristics of the host lipid bilayer. After the virus buds, the maturation process begins. Within the virion, protease, another enzyme unique to HIV, begins cleaving a large precursor polypeptide (gag-pol) into functional proteins that are necessary to produce a complete virus. Without this enzyme, the virion is immature and unable to infect other cells.
The characteristics of viral replication and pathogenesis exhibit three general phases: acute, chronic, and terminal (AIDS).36,37 Initial rounds of HIV replication during acute infection take place largely in the mucosal CD4+ CCR5+ T cell pools in the gut resulting in a massive CD4 T-cell depletion in these tissues.36,37 Cells are destroyed by various mechanisms, including cell lysis from newly budding virions, cytotoxic T-lymphocyte–induced cell killing, and induction of apoptosis. Following this destruction of the mucosal CD4 T cell pool, which lasts for 2 to 3 weeks, a state of heightened immune activation ensues during the chronic infection phase, which can last for several years. The activated state is characterized by high levels of activation markers on circulating T cells and proinflammatory cytokines and may result from HIV antigen as well as translocation of microbial antigens from the T-cell depleted gut mucosa. Heightened activation enables further HIV replication and ultimately leads to continued depletion of CD4+ CCR5+ T cells. HIV-1 exhibits a very high turnover rate during this chronic phase, with an estimated 10 billion new viruses produced each day. More than 99% of these viruses are produced in newly infected activated cells. Nevertheless, the immune system is able to operate well enough during the chronic phase to prevent overt OIs that herald AIDS. Eventually, the depletion of CD4 cells and the continuous cellular activation leads to a final collapse of the immune system, or AIDS. HIV may use CXCR4 coreceptor during this last phase of infection and these viruses infect a broader range of CD4 cells (naïve and central-memory) speeding the disease progression. It is this unrelenting destruction of CD4 cells that causes the profoundly compromised immune system and AIDS.36,37
CLINICAL PRESENTATION
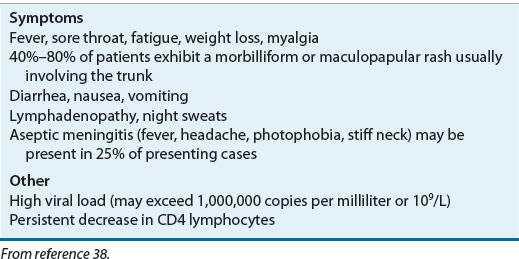
![]() Clinical presentation of primary HIV infection varies, but most patients (40% to 90%) have an acute retroviral syndrome or mononucleosis-like illness (Table 103-2).38 Symptoms often last 2 weeks, and hospitalization may be required for 15% of patients. Primary infection is associated with a high viral load (>106 copies/mL [>109/L]) and a precipitous drop in CD4 cells. After several weeks an immune response is mounted, the amount of HIV RNA in plasma falls substantially, CD4 cells rebound slightly, and symptoms resolve gradually. However, as described above, this clinically latent period is not virologically latent because HIV replication is continuous (∼10 billion viruses per day) and immune system destruction is ongoing. A steady decrease in CD4 cells is the most measurable aspect of this immune system deterioration. Plasma viral load, on the other hand, will appear to have stabilized at a particular level or “set point.” The set point that is established correlates directly with the time to AIDS and morbidity. The Multicenter AIDS Cohort Study measured viral load in 181 HIV-positive men and followed them for as long as 11 years. The mortality rates within 5 years for those with a viral load below 4530 copies/mL (4.53 × 106/L) was 5% compared with 49% for those with a viral load above 36,270 copies/mL (36.27 × 106/L).39 Thus, a higher viral set point is associated with poorer prognosis. Not all individuals infected with HIV progress to AIDS—these so-called “long-term nonprogressors” may be infected with a defective virus (e.g., nef-deficient HIV) or may have an intrinsic ability to resist infection (e.g., CCR5 mutation).33
Clinical presentation of primary HIV infection varies, but most patients (40% to 90%) have an acute retroviral syndrome or mononucleosis-like illness (Table 103-2).38 Symptoms often last 2 weeks, and hospitalization may be required for 15% of patients. Primary infection is associated with a high viral load (>106 copies/mL [>109/L]) and a precipitous drop in CD4 cells. After several weeks an immune response is mounted, the amount of HIV RNA in plasma falls substantially, CD4 cells rebound slightly, and symptoms resolve gradually. However, as described above, this clinically latent period is not virologically latent because HIV replication is continuous (∼10 billion viruses per day) and immune system destruction is ongoing. A steady decrease in CD4 cells is the most measurable aspect of this immune system deterioration. Plasma viral load, on the other hand, will appear to have stabilized at a particular level or “set point.” The set point that is established correlates directly with the time to AIDS and morbidity. The Multicenter AIDS Cohort Study measured viral load in 181 HIV-positive men and followed them for as long as 11 years. The mortality rates within 5 years for those with a viral load below 4530 copies/mL (4.53 × 106/L) was 5% compared with 49% for those with a viral load above 36,270 copies/mL (36.27 × 106/L).39 Thus, a higher viral set point is associated with poorer prognosis. Not all individuals infected with HIV progress to AIDS—these so-called “long-term nonprogressors” may be infected with a defective virus (e.g., nef-deficient HIV) or may have an intrinsic ability to resist infection (e.g., CCR5 mutation).33
TABLE 103-2 Clinical Presentation of Primary Human Immunodeficiency Virus Infection in Adults
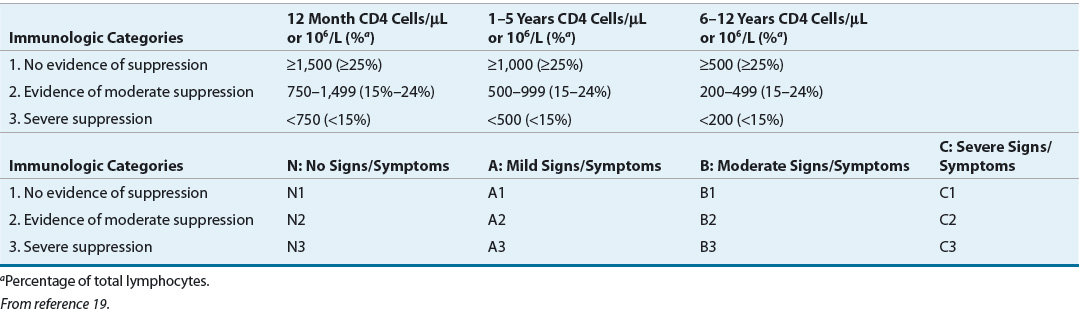
Most children born with HIV are asymptomatic. On physical examination, children often present with nonspecific signs, such as lymphadenopathy, hepatomegaly, splenomegaly, failure to thrive, weight loss or unexplained low birth weight (in prenatally exposed infants), and fever of unknown origin.40 Laboratory findings include anemia, hypergammaglobulinemia (primarily immunoglobulin [Ig] A and IgM), altered mononuclear cell function, and altered T-cell subset ratios. Of note, the normal range for CD4 cell counts in young children is much different from the range in adults (Table 103-3). Children have different susceptibility and/or exposures to OIs compared with adults.40 Bacterial infections, including Streptococcus pneumoniae, Salmonella spp., and Mycobacterium tuberculosis, may be more prevalent in children with AIDS than in adults with the disease. Kaposi’s sarcoma is rare in children. Children with HIV infection may develop lymphocytic interstitial pneumonitis without evidence of P. jirovecii or other pathogens on lung biopsy. Some children (∼25%) will progress to AIDS rapidly within the first year of life. A presentation of serious OIs such as P. jirovecii pneumonia, encephalopathy, failure to thrive, and a precipitous drop in CD4 cells are common in these infants.40 The current CDC pediatric AIDS surveillance definition (see Table 103-3) excludes children with congenital or perinatally acquired cytomegalovirus or other identified causes of congenital immunodeficiency; laboratory-confirmed HIV-infection is required.19 General management of the HIV-infected child involves principles similar to those used for the adult: ART, treatment and prophylaxis of OIs, and supportive care.41,42
TABLE 103-3 Centers for Disease Control and Prevention 2008 Revised Classification System for Human Immunodeficiency Virus Infection in Children Younger Than 13 Years
TREATMENT
Desired Outcome
![]() The central goals of ART are to decrease morbidity and mortality, improve quality of life, restore and preserve immune function, and prevent further transmission. The most important and effective way to achieve these goals is maximal suppression of HIV replication, which is interpreted as plasma HIV RNA less than the lower limit of quantitation (i.e., undetectable; usually <50 copies/mL [<50 × 103/L]).30 Such a profound reduction in HIV RNA is associated with reduced transmissions and long-term response to therapy (i.e., durability), as well as increases in CD4 lymphocytes that closely correlates with a reduced risk for developing OIs. While undetectable HIV RNA almost always corresponds with a rise in CD4 lymphocytes, some patients respond virologically or immunologically without the other.30
The central goals of ART are to decrease morbidity and mortality, improve quality of life, restore and preserve immune function, and prevent further transmission. The most important and effective way to achieve these goals is maximal suppression of HIV replication, which is interpreted as plasma HIV RNA less than the lower limit of quantitation (i.e., undetectable; usually <50 copies/mL [<50 × 103/L]).30 Such a profound reduction in HIV RNA is associated with reduced transmissions and long-term response to therapy (i.e., durability), as well as increases in CD4 lymphocytes that closely correlates with a reduced risk for developing OIs. While undetectable HIV RNA almost always corresponds with a rise in CD4 lymphocytes, some patients respond virologically or immunologically without the other.30
General Approach to Treatment
![]() Prior to 1996, HIV infection was treated with one or two nucleoside analog reverse transcriptase inhibitors (NRTI), which were generally not effective at controlling viremia. Thus, the mainstay of treatment was pharmacologic management of OIs and palliative care. At that time, the prognosis for HIV infection was dire and most patients were disabled and eventually died. In 1995, HIV protease inhibitors (PIs) were introduced followed by NNRTIs, and a new paradigm in HIV treatment was born. Combinations of three active antiretroviral agents from two pharmacologic classes were shown to profoundly inhibit HIV replication to undetectable levels, prevent and reverse immune deficiency, and substantially decrease morbidity and mortality—constituting the ART era.43 At the same time, multiple other major medical advances were introduced, such as the discovery that HIV establishes a long-lived reservoir in chronically infected cells, and the viral load test (plasma HIV-RNA). With this backdrop of dramatic changes, in 1997 the National Institutes of Health Office of AIDS Research convened a panel to define the scientific principles that might serve as a guide for the clinical use of antiretroviral agents.44 The 11 principles presented here are an amalgamation of knowledge of the life cycle of HIV, the consequences of HIV replication, clinical trials of antiretroviral agents, and scientific opinion. These foundational principles are still relevant today.
Prior to 1996, HIV infection was treated with one or two nucleoside analog reverse transcriptase inhibitors (NRTI), which were generally not effective at controlling viremia. Thus, the mainstay of treatment was pharmacologic management of OIs and palliative care. At that time, the prognosis for HIV infection was dire and most patients were disabled and eventually died. In 1995, HIV protease inhibitors (PIs) were introduced followed by NNRTIs, and a new paradigm in HIV treatment was born. Combinations of three active antiretroviral agents from two pharmacologic classes were shown to profoundly inhibit HIV replication to undetectable levels, prevent and reverse immune deficiency, and substantially decrease morbidity and mortality—constituting the ART era.43 At the same time, multiple other major medical advances were introduced, such as the discovery that HIV establishes a long-lived reservoir in chronically infected cells, and the viral load test (plasma HIV-RNA). With this backdrop of dramatic changes, in 1997 the National Institutes of Health Office of AIDS Research convened a panel to define the scientific principles that might serve as a guide for the clinical use of antiretroviral agents.44 The 11 principles presented here are an amalgamation of knowledge of the life cycle of HIV, the consequences of HIV replication, clinical trials of antiretroviral agents, and scientific opinion. These foundational principles are still relevant today.
1. Ongoing HIV replication leads to immune system damage and progression to AIDS. HIV infection is always harmful, and true long-term survival free of clinically significant immune dysfunction is unusual.
2. Plasma HIV RNA levels indicate the magnitude of HIV replication and its associated rate of CD4 cell destruction, whereas CD4 cell counts indicate the extent of HIV-induced immune damage already suffered. Regular periodic measurement of plasma HIV RNA levels and CD4 cell counts is necessary to determine the risk of disease progression in an HIV-infected individual and to determine when to initiate or modify antiretroviral treatment regimens.
3. Because rates of disease progression differ among individuals, treatment decisions should be individualized by level of risk indicated by plasma HIV RNA levels and CD4 cell counts.
4. Use of potent combination ART to suppress HIV replication to below the levels of detection of sensitive plasma HIV RNA assays limits the potential for selection of antiretroviral-resistant HIV variants, the major factor limiting the ability of antiretroviral drugs to inhibit virus replication and delay disease progression. Therefore, maximum achievable suppression of HIV replication should be the goal of therapy.
5. The most effective means for accomplishing durable suppression of HIV replication is simultaneous initiation of combinations of effective anti-HIV drugs with which the patient has not been treated previously and that are not cross-resistant with antiretroviral agents with which the patient has been treated previously.
6. Each of the antiretroviral drugs used in combination therapy regimens always should be used according to optimal schedules and dosages.
7. The available effective antiretroviral drugs are limited in number and mechanism of action, and cross-resistance between specific drugs has been documented. Therefore, any change in ART increases future therapeutic constraints.
8. Women should receive optimal ART regardless of pregnancy status.
9. The same principles of ART apply to both HIV-infected children and adults, although treatment of HIV-infected children involves unique pharmacologic, virologic, and immunologic considerations.
10. Persons with acute primary HIV infections should be treated with combination ART to suppress virus replication to levels below the limit of detection of sensitive plasma HIV RNA assays.
11. HIV-infected persons, even those with viral loads below detectable limits, should be considered infectious and should be counseled to avoid sexual and drug-use behaviors that are associated with transmission or acquisition of HIV and other infectious pathogens.
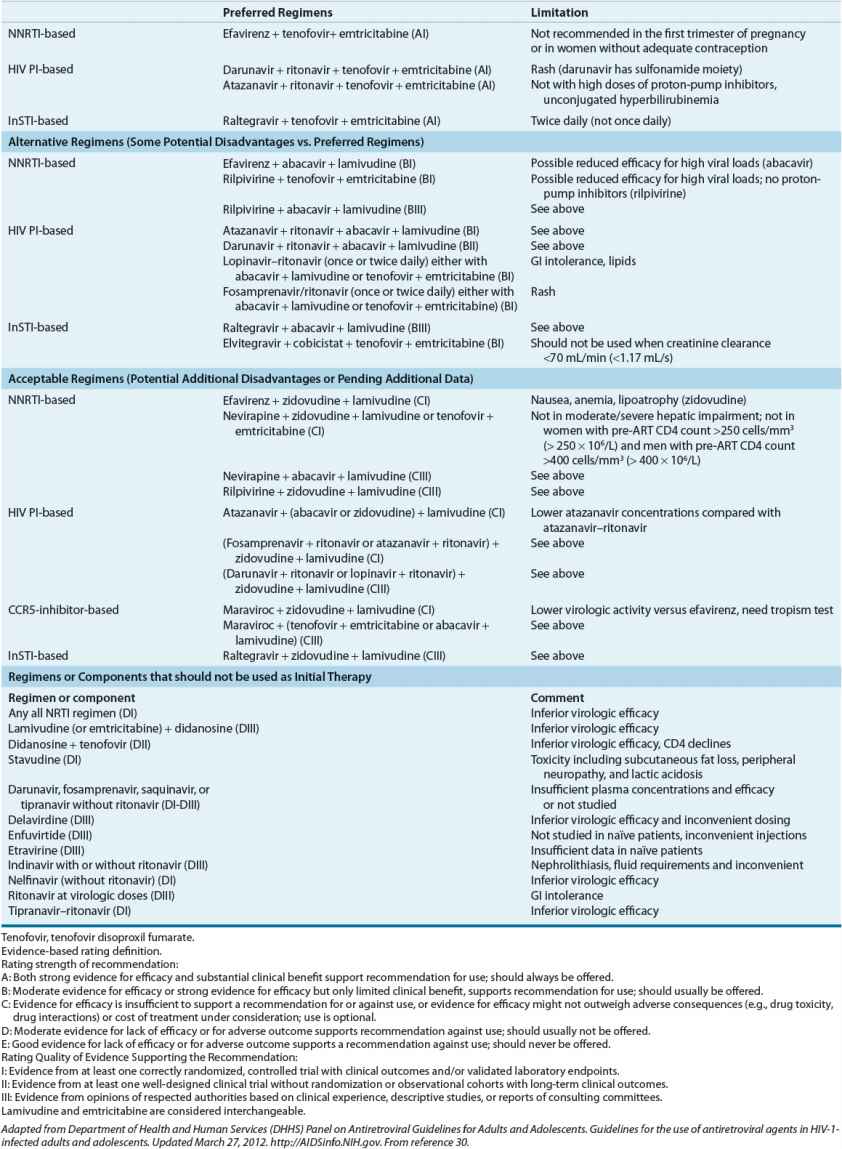
The extent to which these 11 principles will continue to stand the test of time is unknown; new information on the pathogenesis and treatment of HIV accrues constantly. One continuing source of controversy is whether to treat patients with acute HIV infection. As of October 2012, 27 distinct antiretroviral compounds have been approved by the FDA; two (amprenavir and zalcitabine) have since been removed from the market. Table 103-4 presents the state of the art for treatment of HIV-infected individuals as of October 2012.30 Treatment is recommended for all HIV-infected persons with a CD4 lymphocyte count below 500 cells/mm3 (500 × 106/L). Many clinicians would also favor starting therapy in asymptomatic patients with CD4 counts above 500 cells/mm3 (500 × 106/L). Other indications for therapy at any CD4 count include pregnancy, history of AIDS-defining illness, HIV-associated nephropathy, or HIV/hepatitis B virus coinfection.
TABLE 103-4 Treatment of Human Immunodeficiency Virus Infection: Antiretroviral Regimens Recommended in Antiretroviral-Naïve Persons
Clinical Controversy…
The optimal time to initiate therapy in chronic HIV infection has been a matter of debate over the last 15 years. The challenges of life-long ART must be balanced against the higher relative risk of ongoing HIV transmissions and disease progression in asymptomatic HIV-infected individuals when ART is delayed. Randomized trials demonstrate that deaths and disease progression are higher if therapy is delayed until CD4 cells fall below 350 cells/mm3 (350 × 106/L).7,30,45 Epidemiological studies show significantly higher mortality if treatment is delayed in those with a CD4 count of 351 to 500 cells/mm3 (351 × 106 to 500 × 106/L) or CD4 counts >500 cells/mm3 (>500 × 106/L) compared with deferring therapy until CD4 counts drop to lower levels.46 Other epidemiological studies show similar benefits in terms of the composite endpoint of disease progression and death for earlier initiation of therapy.47,48 Together, these studies support early initiation of ART in patients who are ready and willing to commit to life-long treatment including an understanding of its risks and benefits and the need to maintain a high level of adherence. Healthcare professionals involved in the care of HIV-infected persons must consult the most current literature on the principles and strategies for therapy. Better patient outcomes are demonstrated when clinicians have significant HIV expertise. An excellent source for information on treatment guidelines, which is regularly updated, is available at www.AIDSinfo.NIH.gov. Additional guidelines and electronic resources for HIV clinicians are provided in reference 24.
Clinical Controversy…
Pharmacologic Therapy
Conceptually the four primary methods of therapeutic intervention against HIV are direct inhibition of chronic viral replication or prevention of HIV acquisition including as virucidal topical formulations (chemicals that destroy intact viruses) to prevent HIV infection, vaccination to stimulate a more effective immune response, and restoration of the immune system with immunomodulators; the latter three approaches are mostly investigational. Several approaches for an HIV vaccine are in development, including whole killed virus, subunit and peptide vaccination, recombinant live vector, and naked DNA delivery. A randomized placebo controlled trial demonstrated a modest 30% reduction in HIV transmission in a modified-intention to treat analysis of ALVAC-HIV plus AIDSVAX vaccine in 16,402 volunteers.49 The modified analysis excluded subjects who were found to be HIV-infected prior to randomization. However, the efficacy difference was not significant in the per-protocol analysis. Therefore, the findings must be considered tentative until more definitive data become available. Overall, progress has been slow for the vaccine field. Genetic variability in HIV and a nascent understanding of the role of the immune system in suppressing viral replication are significant barriers to the development of an effective HIV vaccine with long-lasting and protective immunity. Immunomodulators, such as aldesleukin (interleukin-2), provide mild benefits in terms of increased CD4 cells; however, aldesleukin is also associated with significant toxicities and no apparent clinical benefit.50 Additional immunotherapies are in earlier phases of study. Topical virucidal or antiretroviral drug formulations for use vaginally or rectally to prevent sexual transmission of HIV are in various phases of development. Vaginal application of tenofovir 1% gel before and after intercourse reduced HIV infection by 39% in women.12 However, another study showed that daily tenofovir 1% gel vaginally did not reduce HIV acquisition, indicating that additional trials will be needed before licensing decisions are made.
Antiretroviral Agents
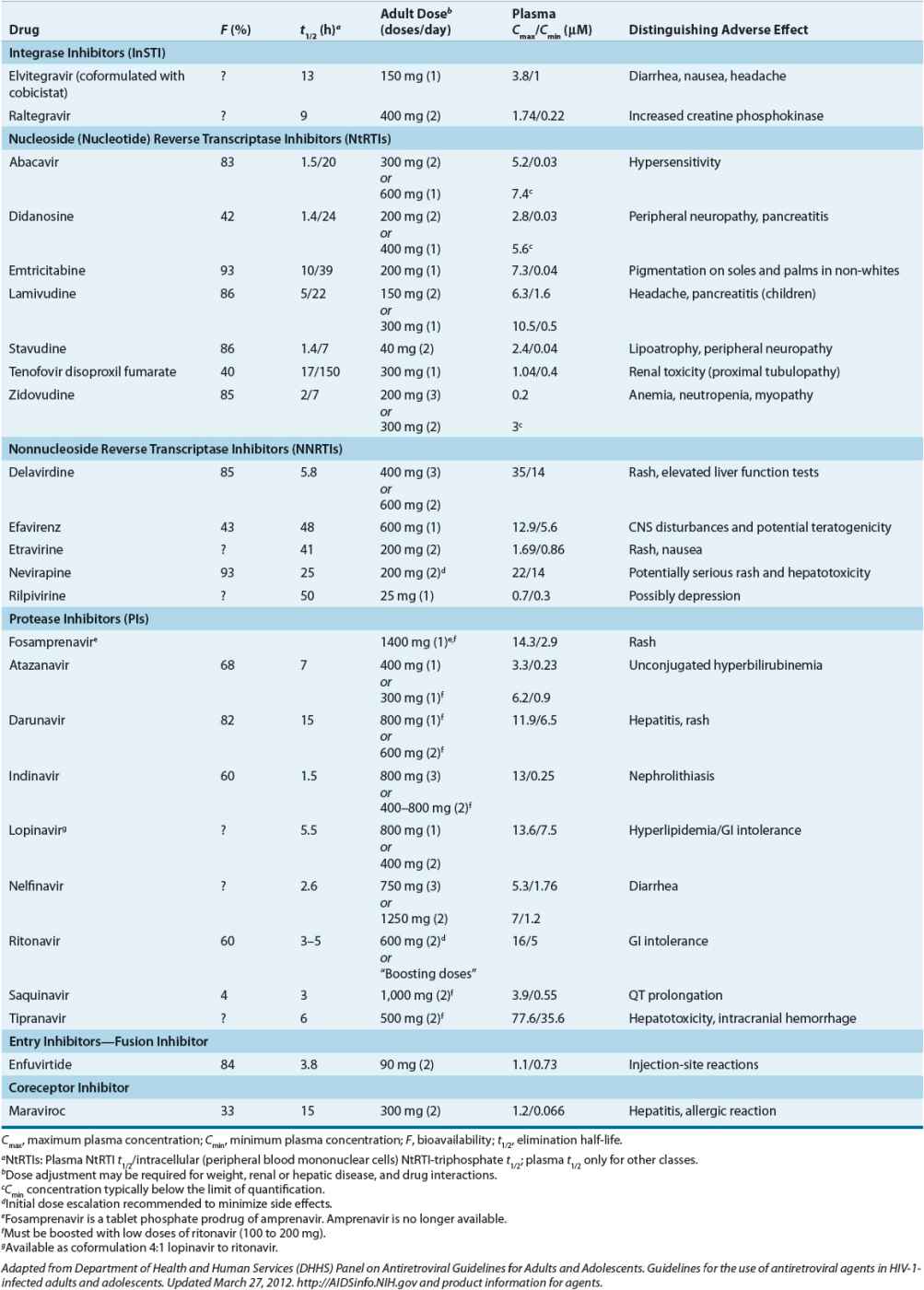
Direct inhibition of viral replication with combinations of potent antiretroviral agents has been the most clinically successful strategy. Four general classes of drugs are used today: entry inhibitors, reverse transcriptase inhibitors, InSTIs, and HIV PIs (Table 103-5).30 Reverse transcriptase inhibitors consist of two classes: those that are chemical derivatives of purine- and pyrimidine-based nucleosides and nucleotides (nucleoside/nucleotide reverse transcriptase inhibitors [NRTIs]) and those that are not (nonnucleoside reverse transcriptase inhibitors [NNRTIs]). NRTIs include the thymidine analogs stavudine (d4T) and zidovudine (AZT or ZDV); the deoxycytidine analogs emtricitabine (FTC) and lamivudine (3TC); the deoxyguanosine analog abacavir sulfate (ABC); and the deoxyadenosine analogs of which didanosine (ddI) is an inosine derivative and tenofovir disoproxil fumarate (TDF) is a deoxyadenosine-monophosphate nucleotide analog (a nucleotide is a nucleoside with one or more phosphates). Note that drug abbreviations are provided here and below for reference, but their use is discouraged because they may lead to prescribing or administration errors. As a class, the NRTIs require phosphorylation to the 5’-triphosphate moiety to become pharmacologically active. Intracellular phosphorylation occurs by cytoplasmic or mitochondrial kinases and phosphotransferases (not viral kinases). The 5’-triphosphate moiety acts in two ways: (a) it competes with endogenous deoxyribonucleotides for the catalytic site of reverse transcriptase, and (b) it prematurely terminates DNA elongation, if taken up and incorporated, as it lacks the requisite 3’-hydroxyl for sugar-phosphate linking.42 Although NRTI triphosphates (or diphosphate for tenofovir) are specific for HIV reverse transcriptase, their adverse effects may be caused in part by inhibition of mitochondrial DNA or RNA synthesis.51 Toxicities include peripheral neuropathy, pancreatitis, lipoatrophy (subcutaneous fat loss), myopathy, anemia, and rarely life-threatening lactic acidosis with fatty liver.52 Use of stavudine and didanosine has declined in favor of more tolerable NRTIs (e.g., emtricitabine, lamivudine, and tenofovir).30 Emtricitabine, lamivudine, and tenofovir are active against hepatitis B virus, and a combination of these agents should be used in HIV–hepatitis B coinfected patients. With some exceptions (e.g., abacavir), NRTIs are mainly eliminated by the kidney and dose adjustments are required for renal insufficiency (and abacavir should not be used in advanced hepatic impairment). Resistance has been reported for all NRTIs, including cross-resistance within the class as multiple and/or specific mutations accrue.53
TABLE 103-5 Selected Pharmacologic Characteristics of Antiretroviral Compounds