42
Hormone Action & Signal Transduction
OBJECTIVES
After studying this chapter, you should be able to:
![]() Explain the roles of stimulus, hormone release, signal generation, and effector response in a variety of hormone-regulated physiological processes.
Explain the roles of stimulus, hormone release, signal generation, and effector response in a variety of hormone-regulated physiological processes.
![]() Explain the role of receptors and GTP-binding G-proteins in hormone signal transduction, particularly with regard to the generation of second messengers.
Explain the role of receptors and GTP-binding G-proteins in hormone signal transduction, particularly with regard to the generation of second messengers.
![]() Appreciate the complex patterns of signal transduction pathway cross-talk in mediating complicated physiological outputs.
Appreciate the complex patterns of signal transduction pathway cross-talk in mediating complicated physiological outputs.
![]() Understand the key roles that protein-ligand, protein-protein, protein post-translational modification (eg., phosphorylation and acetylation), and protein-DNA interactions play in mediating hormone-directed physiological processes.
Understand the key roles that protein-ligand, protein-protein, protein post-translational modification (eg., phosphorylation and acetylation), and protein-DNA interactions play in mediating hormone-directed physiological processes.
![]() Appreciate that hormone-modulated receptors, second messengers, and associated signaling molecules represent a rich source of potential drug target development given their key roles in the regulation of physiology.
Appreciate that hormone-modulated receptors, second messengers, and associated signaling molecules represent a rich source of potential drug target development given their key roles in the regulation of physiology.
BIOMEDICAL IMPORTANCE
The homeostatic adaptations an organism makes to a constantly changing environment are in large part accomplished through alterations of the activity and amount of proteins. Hormones provide a major means of facilitating these changes. A hormone-receptor interaction results in generation of an intracellular signal that can either regulate the activity of a select set of genes, thereby altering the amount of certain proteins in the target cell, or affect the activity of specific proteins, including enzymes and transporter or channel proteins. The signal can influence the location of proteins in the cell and can affect general processes such as protein synthesis, cell growth, and replication, often through effects on gene expression. Other signaling molecules—including cytokines, interleukins, growth factors, and metabolites—use some of the same general mechanisms and signal transduction pathways. Excessive, deficient, or inappropriate production and release of hormones and of these other regulatory molecules are major causes of disease. Many pharmacotherapeutic agents are aimed at correcting or otherwise influencing the pathways discussed in this chapter.
HORMONES TRANSDUCE SIGNALS TO AFFECT HOMEOSTATIC MECHANISMS
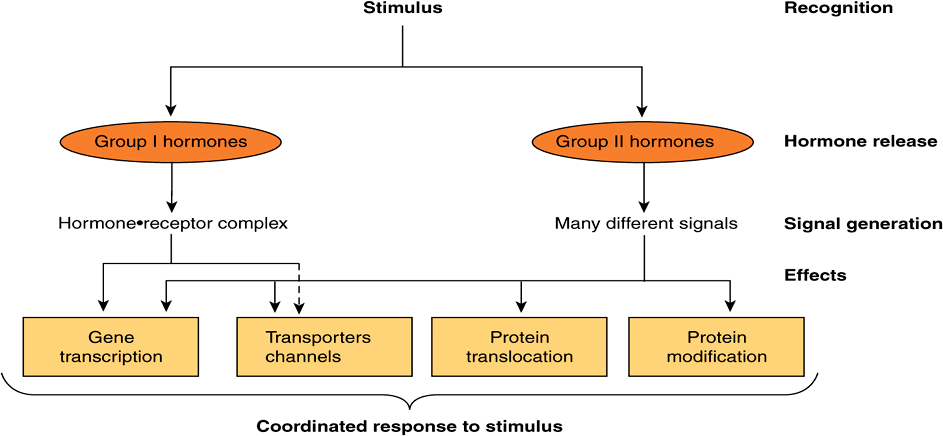
The general steps involved in producing a coordinated response to a particular stimulus are illustrated in Figure 42–1. The stimulus can be a challenge or a threat to the organism, to an organ, or to the integrity of a single cell within that organism. Recognition of the stimulus is the first step in the adaptive response. At the organismic level, this generally involves the nervous system and the special senses (sight, hearing, pain, smell, and touch). At the organismic or cellular level, recognition involves physicochemical factors such as pH, O2 tension, temperature, nutrient supply, noxious metabolites, and osmolarity. Appropriate recognition results in the release of one or more hormones that will govern generation of the necessary adaptive response. For purposes of this discussion, the hormones are categorized as described in Chapter 41, ie, based on the location of their specific cellular receptors and the type of signals generated. Group I hormones interact with an intracellular receptor and group II hormones with receptor recognition sites located on the extracellular surface of the plasma membrane of target cells. The cytokines, interleukins, and growth factors should also be considered in this latter category. These molecules, of critical importance in homeostatic adaptation, are hormones in the sense that they are produced in specific cells, have the equivalent of autocrine, paracrine, and endocrine actions, bind to cell surface receptors, and activate many of the same signal transduction pathways employed by the more traditional group II hormones.
FIGURE 42–1 Hormonal involvement in responses to a stimulus. A challenge to the integrity of the organism elicits a response that includes the release of one or more hormones. These hormones generate signals at or within target cells, and these signals regulate a variety of biologic processes that provide for a coordinated response to the stimulus or challenge. See Figure 42–8 for a specific example.
SIGNAL GENERATION
The Ligand-Receptor Complex Is the Signal for Group I Hormones
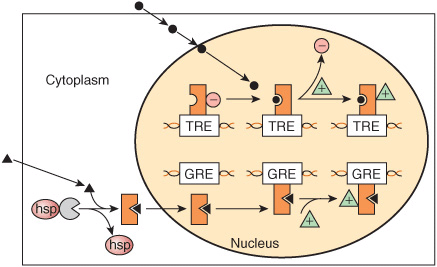
The lipophilic group I hormones diffuse through the plasma membrane of all cells but only encounter their specific, high-affinity intracellular receptors in target cells. These receptors can be located in the cytoplasm or in the nucleus of target cells. The hormone-receptor complex first undergoes an activation reaction. As shown in Figure 42–2, receptor activation occurs by at least two mechanisms. For example, glucocorticoids diffuse across the plasma membrane and encounter their cognate receptor in the cytoplasm of target cells. Ligand-receptor binding results in a conformational change in the receptor leading to the dissociation of heat shock protein 90 (hsp90). This step appears to be necessary for subsequent nuclear localization of the glucocorticoid receptor. This receptor also contains a nuclear localization sequence that is now free to assist in the translocation from cytoplasm to nucleus. The activated receptor moves into the nucleus (Figure 42–2) and binds with high affinity to a specific DNA sequence called the hormone response element (HRE). In the case illustrated, this is a glucocorticoid response element, or GRE. Consensus sequences for HREs are shown in Table 42-1. The DNA-bound, liganded receptor serves as a high-affinity binding site for one or more coactivator proteins and accelerated gene transcription typically ensues when this occurs. By contrast, certain hormones such as the thyroid hormones and retinoids diffuse from the extracellular fluid across the plasma membrane and go directly into the nucleus. In this case, the cognate receptor is already bound to the HRE (the thyroid hormone response element [TRE], in this example). However, this DNA-bound receptor fails to activate transcription because it exists in complex with a corepressor. Indeed, this receptor-corepressor complex serves as an active repressor of gene transcription. The association of ligand with these receptors results in dissociation of the corepressor(s). The liganded receptor is now capable of binding one or more co-activators with high affinity, resulting in the recruitment of RNA polymerase II and the GTFs and activation of gene transcription. The relationship of hormone receptors to other nuclear receptors and to coregulators is discussed in more detail below.
FIGURE 42–2 Regulation of gene expression by two different class I hormones, thyroid hormone and glucocorticoids. The hydrophobic steroid hormones readily gain access to the cytoplasmic compartment of target cells by diffusion through the plasma membrane. Glucocorticoid hormones (solid triangles) encounter their cognate receptor (GR) in the cytoplasm, where GR exists in a complex with heat shock protein 90 (hsp). Ligand binding causes dissociation of hsp and a conformational change of the receptor. The receptor-ligand complex then traverses the nuclear membrane and binds to DNA with specificity and high affinity at a glucocorticoid response element (GRE). This event affects the architecture of a number of transcription coregulators (green triangles), and enhanced transcription ensues. By contrast, thyroid hormones and retinoic acid (•) directly enter the nucleus, where their cognate heterodimeric (TR-RXR; see Figure 42–12) receptors are already bound to the appropriate response elements with an associated transcription repressor complex (red circles). Hormone-receptor binding occurs, which again induces conformational changes in receptor leading to a reorganization of receptor (TR)-coregulator interactions (ie, molecules such as N-CoR or SMRT [see Table 42-6]). Ligand binding results in dissociation of the repressor complex from the receptor, allowing an activator complex, consisting of the TR-TRE and coactivator, to assemble. The gene is then actively transcribed.
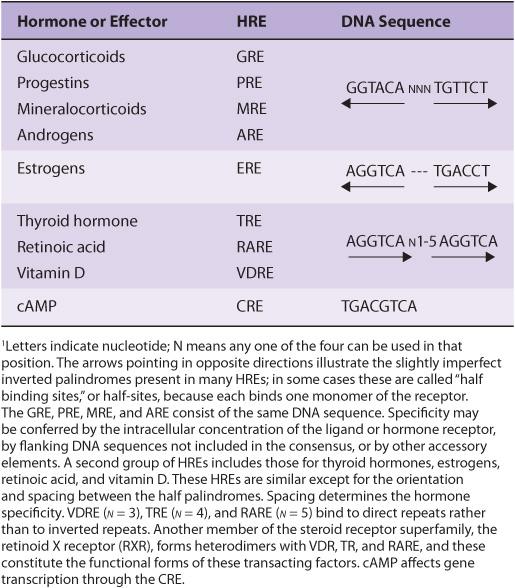
TABLE 42–1 The DNA Sequences of Several Hormone Response Elements (HREs)1
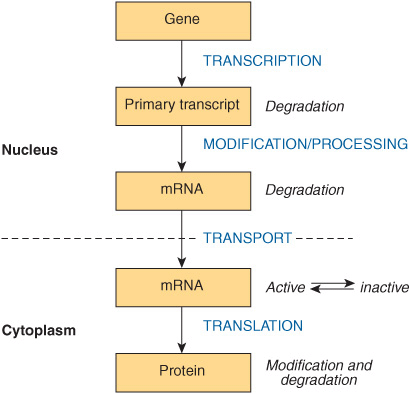
By selectively affecting gene transcription and the consequent production of appropriate target mRNAs, the amounts of specific proteins are changed and metabolic processes are influenced. The influence of each of these hormones is quite specific; generally, a given hormone affects <1% of the genes, mRNA, or proteins in a target cell; sometimes only a few are affected. The nuclear actions of steroid, thyroid, and retinoid hormones are quite well defined. Most evidence suggests that these hormones exert their dominant effect on modulating gene transcription, but they—and many of the hormones in the other classes discussed below—can act at any step of the “information pathway,” as illustrated in Figure 42–3. to control specific gene expression and, ultimately, a biological response. Direct actions of steroids in the cytoplasm and on various organelles and membranes have also been described. Recently, microRNAs have been implicated in mediating some of the diverse actions of the peptide hormone insulin.
FIGURE 42–3 The “information pathway.” Information flows from the gene to the primary transcript to mRNA to protein. Hormones can affect any of the steps involved and can affect the rates of processing, degradation, or modification of the various products.
GROUP II (PEPTIDE & CATECHOLAMINE) HORMONES HAVE MEMBRANE RECEPTORS & USE INTRACELLULAR MESSENGERS
Many hormones are water soluble, have no transport proteins (and therefore have a short plasma half-life), and initiate a response by binding to a receptor located in the plasma membrane (Tables 41-3 and 41-4). The mechanism of action of this group of hormones can best be discussed in terms of the intracellular signals they generate. These signals include cAMP (cyclic AMP; 3’,5’-adenylic acid; see Figure 19–5), a nucleotide derived from ATP through the action of adenylyl cyclase; cGMP, a nucleotide formed by guanylyl cyclase; Ca2+; and phosphatidylinositides; such small molecules are termed second messengers as their synthesis is triggered by the presence of the primary hormone (molecule) binding its receptor. Many of these second messengers affect gene transcription, as described in the previous paragraph; but they also influence a variety of other biologic processes, as shown in Figure 42–3.
G Protein-Coupled Receptors
Many of the group II hormones bind to receptors that couple to effectors through a GTP-binding protein (G-proteins) intermediary. These receptors typically have seven hydrophobic plasma membrane-spanning domains. This is illustrated by the seven interconnected helices extending through the lipid bilayer in Figure 42–4. Receptors of this class, which signal through guanine nucleotide-bound protein intermediates, are known as G protein-coupled receptors (GPCRs). To date, hundreds of G protein-linked receptor genes have been identified; this represents the largest family of cell surface receptors in humans. A wide variety of responses are mediated by the GPCRs.
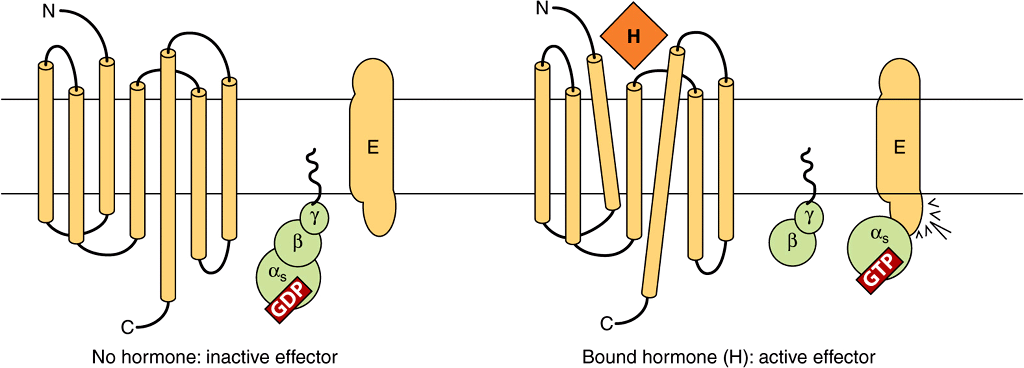
FIGURE 42–4 Components of the hormone receptor–G protein effector system. Receptors that couple to effectors through G proteins (GPCR) typically have seven membrane-spanning domains. In the absence of hormone (left), the heterotrimeric G-protein complex (α,β,γ) is in an inactive guanosine diphosphate (GDP)-bound form and is probably not associated with the receptor. This complex is anchored to the plasma membrane through prenylated groups on the βγ subunits (wavy lines) and perhaps by myristoylated groups on α subunits (not shown). On binding of hormone (H) to the receptor, there is a presumed conformational change of the receptor—as indicated by the tilted membrane spanning domains—and activation of the G-protein complex. This results from the exchange of GDP with guanosine triphosphate (GTP) on the α subunit, after which α and βγ dissociate. The α subunit binds to and activates the effector (E). E can be adenylyl cyclase, Ca2+, Na+, or Cl– channels (αs), or it could be a K+ channel (α1), phospholipase Cβ (αη), or cGMP phosphodiesterase (αt). The βγ subunit can also have direct actions on E. (Modified and reproduced, with permission, from Granner DK in: Principles and Practice of Endocrinology and Metabolism, 2nd ed. Becker KL (editor). Lippincott, 1995.)
cAMP Is the Intracellular Signal for Many Responses
Cyclic AMP was the first intracellular second messenger signal identified in mammalian cells. Several components comprise a system for the generation, degradation, and action of cAMP.
Adenylyl Cyclase
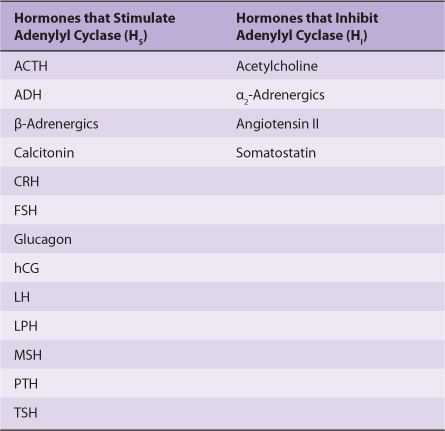
Different peptide hormones can either stimulate (s) or inhibit (i) the production of cAMP from adenylyl cyclase, which is encoded by at least nine different genes (Table 42-2). Two parallel systems, a stimulatory (s) one and an inhibitory (i) one, converge upon a catalytic molecule (C). Each consists of a receptor, Rs or Ri, and a regulatory complex, Gs and Gi. Gs and Gi are each heterotrimeric G-protein composed of α, β, and γ subunits. Since the α subunit in Gs differs from that in Gi, the proteins, which are distinct gene products, are designated αs and αi. The α subunits bind guanine nucleotides. The β and γ subunits are always associated (βγ) and appear to function as a heterodimer. The binding of a hormone to Rs or Ri results in a receptor-mediated activation of G, which entails the exchange of GDP by GTP on α and the concomitant dissociation of βγ from α.
TABLE 42–2 Subclassification of Group II.A Hormones
The α protein has intrinsic GTPase activity. The active form, αs-GTP, is inactivated upon hydrolysis of the GTP to GDP; the trimeric Gs complex (αβγ) is then re-formed and is ready for another cycle of activation. Cholera and pertussis toxins catalyze the ADPribosylation of as, and αi-2 (see Table 42-3), respectively. In the case of αs, this modification disrupts the in trinsic GTPase activity; thus, αs cannot reassociate with βγ and is therefore irreversibly activated. ADP ribosylation of αi-2 prevents the dissociation of αi-2 from βγ, and free αi-2 thus cannot be formed. α activity in such cells is therefore unopposed.
TABLE 42-3 Classes and Functions of Selected G Proteins1
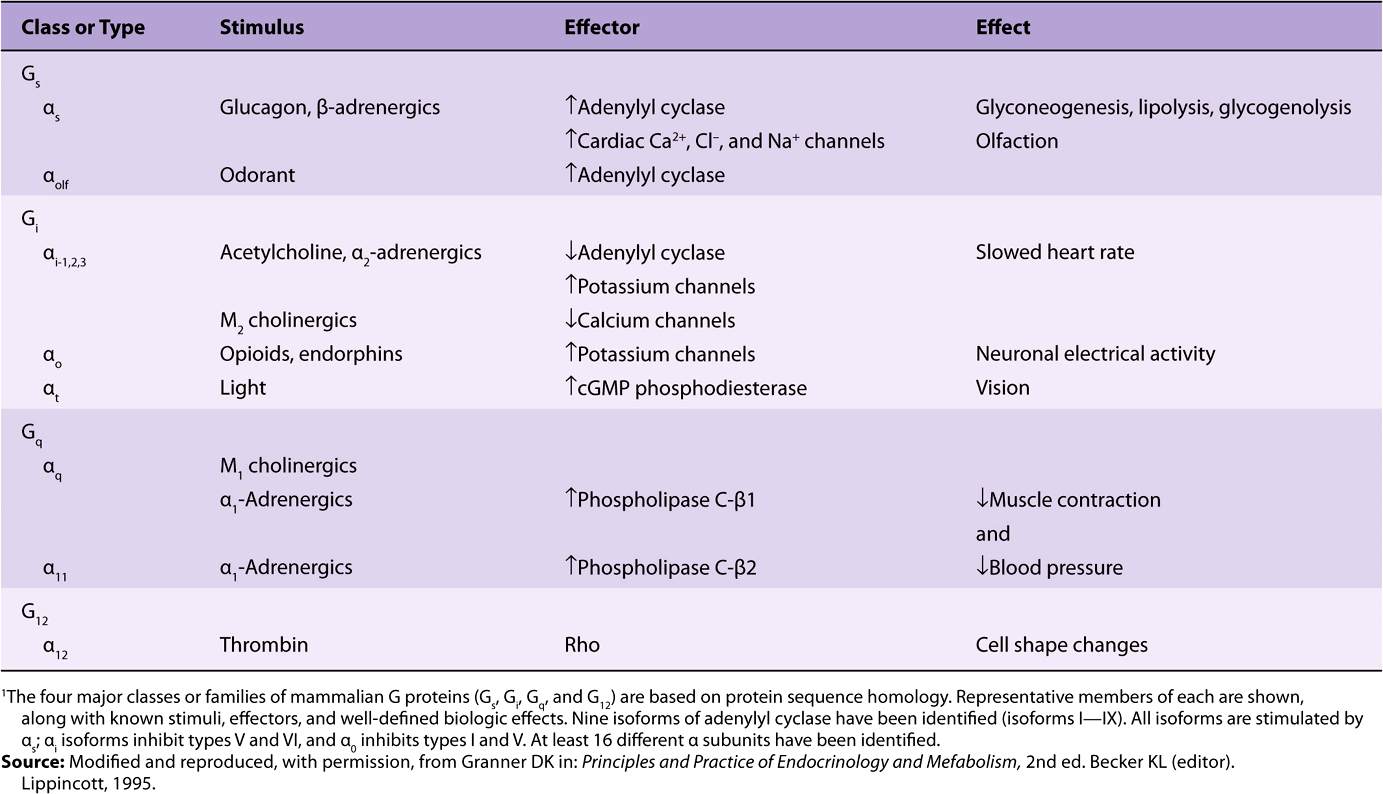
There is a large family of G proteins, and these are part of the superfamily of GTPases. The G protein family is classified according to sequence homology into four subfamilies, as illustrated in Table 42-3. There are 21 α, 5 β, and 8 γ subunit genes. Various combinations of these subunits provide a large number of possible αβγ and cyclase complexes.
The α subunits and the βγ complex have actions independent of those on adenylyl cyclase (see Figure 42–4 and Table 42-3). Some forms of αi, stimulate K+ channels and inhibit Ca2+ channels, and some αs molecules have the opposite effects. Members of the Gq family activate the phospholipase C group of enzymes. The βγ complexes have been associated with K+ channel stimulation and phospholipase C activation. G proteins are involved in many important biologic processes in addition to hormone action. Notable examples include olfaction (αOLF) and vision (αt). Some examples are listed in Table 42-3. GPCRs are implicated in a number of diseases and are major targets for pharmaceutical agents.
Protein Kinase
As discussed in Chapter 38, in prokaryotic cells, cAMP binds to a specific protein called catabolite regulatory protein (CRP) that binds directly to DNA and influences gene expression. By contrast, in eukaryotic cells, cAMP binds to a protein kinase called protein kinase A (PKA), a heterotetrameric molecule consisting of two regulatory subunits (R) and two catalytic subunits (C). cAMP binding results in the following reaction:
![]()
The R2C2 complex has no enzymatic activity, but the binding of cAMP by R induces dissociation of the R-C complex, thereby activating the latter (Figure 42–5). The active C subunit catalyzes the transfer of the γ phosphate of ATP to a serine or threonine residue in a variety of proteins. The consensus phosphorylation sites are -ArgArg/Lys-X-Ser/Thr- and -Arg-Lys-X-X-Ser-, where X can be any amino acid.
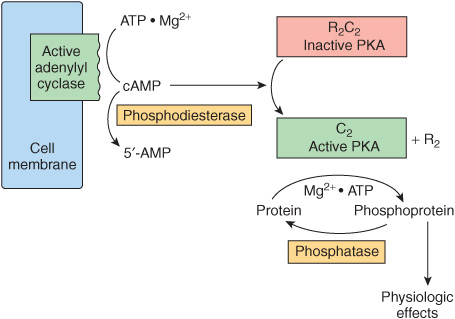
FIGURE 42–5 Hormonal regulation of cellular processes through cAMP-dependent protein kinase (PKA). PKA exists in an inactive form as an R2C2 heterotetramer consisting of two regulatory (R) and two catalytic (C) subunits. The cAMP generated by the action of adenylyl cyclase (activated as shown in Figure 42–4) binds to the regulatory subunit of PKA. This results in dissociation of the regulatory and catalytic subunits and activation of the latter. The active catalytic subunits phosphorylate a number of target proteins on serine and threonine residues. Phosphatases remove phosphate from these residues and thus terminate the physiologic response. A phosphodiesterase can also terminate the response by converting cAMP to 5′-AMP.
Protein kinase activities were originally described as being “cAMP-dependent” or “cAMP-independent.” This classification has changed, as protein phosphorylation is now recognized as being a major regulatory mechanism. Several hundred protein kinases have now been described. The kinases are related in sequence and structure within the catalytic domain, but each is a unique molecule with considerable variability with respect to subunit composition, molecular weight, auto-phosphorylation, Km for ATP, and substrate specificity. Both kinase and protein phosphatase activities can be targeted by interaction with specific kinase binding proteins. In the case of PKA, such targeting proteins are termed AKAPs (A kinase anchoring proteins), they serve as scaffolds, which localize PKA near to substrates thereby focusing PKA activity toward physiological substrates and facilitating spatiotemporal biological regulation while also allowing for common, shared proteins to elicit specific physiological responses. Multiple AKAPs have been described; they can bind PKA and other kinases as well as phosphatases, phosophodiestases (which hydrolyze cAMP), and protein kinase substrates.
The effects of cAMP in eukaryotic cells are all thought to be mediated by protein phosphorylation-dephosphorylation, principally on serine and threonine residues. The control of any of the effects of cAMP, including such diverse processes as steroidogenesis, secretion, ion transport, carbohydrate and fat metabolism, enzyme induction, gene regulation, synaptic transmission, and cell growth and replication, could be conferred by a specific protein kinase, by a specific phosphatase, or by specific substrates for phosphorylation. These substrates help define a target tissue and are involved in defining the extent of a particular response within a given cell. For example, the effects of cAMP on gene transcription are mediated by the protein cyclic AMP response element binding protein (CREB). CREB binds to a cAMP responsive element (CRE) (see Table 42-1) in its nonphosphorylated state and is a weak activator of transcription. When phosphorylated by PKA, CREB binds the coactivator CREB-binding protein CBP/p300 (see below) and as a result is a much more potent transcription activator. CBP and the related p300 contain histone acetyltransferase activities, and hence serve as chromatin-active transcriptional coregulators (Chapters 36, 38). Interestingly, CBP/p300 can also acetylate certain transcription factors thereby stimulating their ability to bind DNA and modulate transcription.
Phosphodiesterases
Actions caused by hormones that increase cAMP concentration can be terminated in a number of ways, including the hydrolysis of cAMP to 5′-AMP by phosphodiesterases (see Figure 42–5). The presence of these hydrolytic enzymes ensures a rapid turnover of the signal (cAMP) and hence a rapid termination of the biologic process once the hormonal stimulus is removed. There are at least 11 known members of the phosphodiesterase family of enzymes. These are subject to regulation by their substrates, cAMP and cGMP; by hormones; and by intracellular messengers such as calcium, probably acting through calmodulin. Inhibitors of phosphodiesterase, most notably methylated xanthine derivatives such as caffeine, increase intracellular cAMP and mimic or prolong the actions of hormones through this signal.
Phosphoprotein Phosphatases
Given the importance of protein phosphorylation, it is not surprising that regulation of the protein dephosphorylation reaction is another important control mechanism (see Figure 42–5). The phosphoprotein phosphatases are themselves subject to regulation by phosphorylation-dephosphorylation reactions and by a variety of other mechanisms, such as protein-protein interactions. In fact, the substrate specificity of the phosphoserine-phosphothreonine phosphatases may be dictated by distinct regulatory subunits whose binding is regulated hormonally. One of the best-studied roles of regulation by the dephosphorylation of proteins is that of glycogen metabolism in muscle. Two major types of phosphoserine-phosphothreonine phosphatases have been described. Type I preferentially dephosphorylates the β subunit of phosphorylase kinase, whereas type II dephosphorylates the α subunit. Type I phosphatase is implicated in the regulation of glycogen synthase, phosphorylase, and phosphorylase kinase. This phosphatase is itself regulated by phosphorylation of certain of its subunits, and these reactions are reversed by the action of one of the type II phosphatases. In addition, two heat-stable protein inhibitors regulate type I phosphatase activity. Inhibitor-1 is phosphorylated and activated by cAMP-dependent protein kinases, and inhibitor-2, which may be a subunit of the inactive phosphatase, is also phosphorylated, possibly by glycogen synthase kinase-3. Phosphatases that attack phosphotyrosine are also important in signal transduction (see Figure 42–8).
cGMP Is Also an Intracellular Signal
Cyclic GMP is made from GTP by the enzyme guanylyl cyclase, which exists in soluble and membrane-bound forms. Each of these isozymes has unique physiologic properties. The atriopeptins, a family of peptides produced in cardiac atrial tissues, cause natriuresis, diuresis, vasodilation, and inhibition of aldosterone secretion. These peptides (eg, atrial natriuretic factor) bind to and activate the membrane-bound form of guanylyl cyclase. This results in an increase of cGMP by as much as 50-fold in some cases, and this is thought to mediate the effects mentioned above. Other evidence links cGMP to vasodilation. A series of compounds, including nitroprusside, nitroglycerin, nitric oxide, sodium nitrite, and sodium azide, all cause smooth muscle relaxation and are potent vasodilators. These agents increase cGMP by activating the soluble form of guanylyl cyclase, and inhibitors of cGMP phosphodiesterase (the drug sildenafil [Viagra], for example) enhance and prolong these responses. The increased cGMP activates cGMP-dependent protein kinase (PKG), which in turn phosphorylates a number of smooth muscle proteins. Presumably, this is involved in relaxation of smooth muscle and vasodilation.
Several Hormones Act Through Calcium or Phosphatidylinositols
Ionized calcium is an important regulator of a variety of cellular processes, including muscle contraction, stimulus-secretion coupling, blood clotting cascade, enzyme activity, and membrane excitability. It is also an intracellular messenger of hormone action.
Calcium Metabolism
The extracellular calcium (Ca2+) concentration is ~5 mmol/L and is very rigidly controlled. Although substantial amounts of calcium are associated with intracellular organelles such as mitochondria and the endoplasmic reticulum, the intracellular concentration of free or ionized calcium (Ca2 +) is very low: 0.05-10 μmol/L. In spite of this large concentration gradient and a favorable trans-membrane electrical gradient, Ca2+ is restrained from entering the cell. A considerable amount of energy is expended to ensure that the intracellular Ca2+ is controlled, as a prolonged elevation of Ca2+ in the cell is very toxic. A Na+/Ca2+ exchange mechanism that has a high-capacity but low-affinity pumps Ca2+ out of cells. There also is a Ca2+/proton ATPase-dependent pump that extrudes Ca2+ in exchange for H+. This has a high affinity for Ca2+ but a low capacity and is probably responsible for fine-tuning cytosolic Ca2+. Furthermore, Ca2+-ATPases pump Ca2+ from the cytosol to the lumen of the endoplasmic reticulum. There are three ways of changing cytosolic Ca2+: (1) Certain hormones (class II.C, Table 41-3) by binding to receptors that are themselves Ca2+ channels, enhance membrane permeability to Ca2 +, and thereby increase Ca2+ influx. (2) Hormones also indirectly promote Ca2+ influx by modulating the membrane potential at the plasma membrane. Membrane depolarization opens voltage-gated Ca2+ channels and allows for Ca2+ influx. (3) Ca2+ can be mobilized from the endoplasmic reticulum, and possibly from mitochondrial pools.
An important observation linking Ca2+ to hormone action involved the definition of the intracellular targets of Ca2+ action. The discovery of a Ca,2+-dependent regulator of phosphodiesterase activity provided the basis for a broad understanding of how Ca2+ and cAMP interact within cells.
Calmodulin
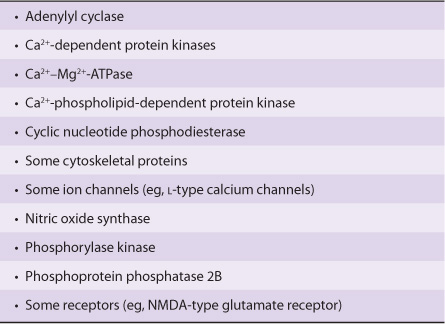
The calcium-dependent regulatory protein is calmodulin, a 17-kDa protein that is homologous to the muscle protein troponin C in structure and function. Calmodulin has four Ca2+ binding sites, and full occupancy of these sites leads to a marked conformational change, which allows calmodulin to activate enzymes and ion channels. The interaction of Ca2+ with calmodulin (with the resultant change of activity of the latter) is conceptually similar to the binding of cAMP to PKA and the subsequent activation of this molecule. Calmodulin can be one of numerous subunits of complex proteins and is particularly involved in regulating various kinases and enzymes of cyclic nucleotide generation and degradation. A partial list of the enzymes regulated directly or indirectly by Ca2+, probably through calmodulin, is presented in Table 42-4.
TABLE 42–4 Enzymes and Proteins Regulated by Calcium or Calmodulin
In addition to its effects on enzymes and ion transport, Ca2+/calmodulin regulates the activity of many structural elements in cells. These include the actin-myosin complex of smooth muscle, which is under β-adrenergic control, and various microfilament-mediated processes in noncontractile cells, including cell motility, cell conformation changes, mitosis, granule release, and endocytosis.
Calcium Is a Mediator of Hormone Action
A role for Ca2+ in hormone action is suggested by the observations that the effect of many hormones is (1) blunted by Ca2+-free media or when intracellular calcium is depleted; (2) can be mimicked by agents that increase cytosolic Ca2+, such as the Ca2+ ionophore A23187; and (3) influences cellular calcium flux. The regulation of glycogen metabolism in liver by vasopressin and β-adrenergic catecholamines provides a good example. This is shown schematically in Figures 19-6 and 19-7.
A number of critical metabolic enzymes are regulated by Ca2+, phosphorylation, or both, including glycogen synthase, pyruvate kinase, pyruvate carboxylase, glycerol-3-phosphate dehydrogenase, and pyruvate dehydrogenase.
Phosphatidylinositide Metabolism Affects Ca2+-Dependent Hormone Action
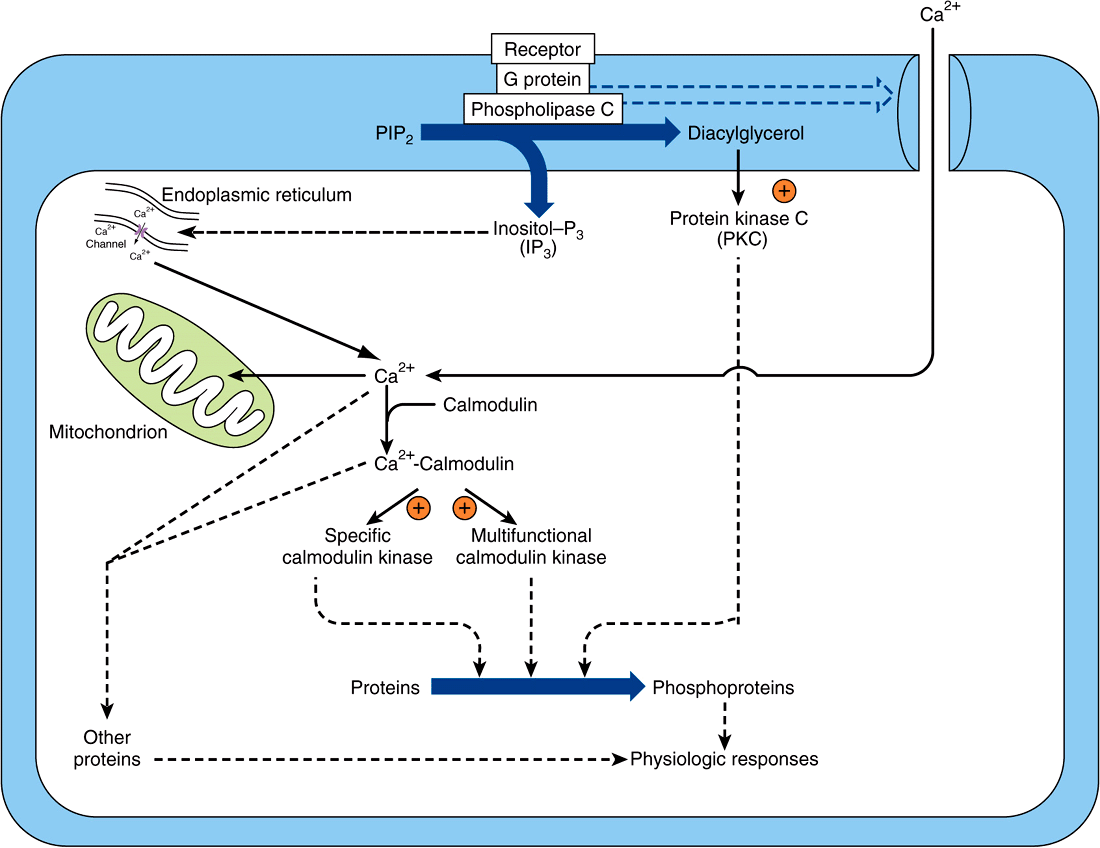
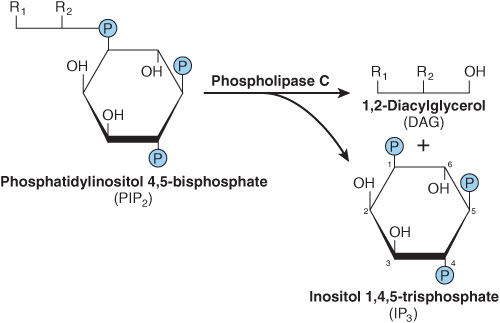
Some signal must provide communication between the hormone receptor on the plasma membrane and the intracellular Ca2+ reservoirs. This is accomplished by products of phosphatidylinositol metabolism. Cell surface receptors such as those for acetylcholine, antidiuretic hormone, and α1-type catecholamines are, when occupied by their respective ligands, potent activators of phospholipase C. Receptor binding and activation of phospholipase C are coupled by the Gq isoforms (Table 42-3 & Figure 42–6). Phospholipase C catalyzes the hydrolysis of phosphatidylinositol 4,5-bisphosphate to inositol trisphosphate (IP3) and 1,2-diacylglycerol (Figure 42–7). Diacylglycerol is itself capable of activating protein kinase C (PKC), the activity of which also depends upon Ca2+. IP3, by interacting with a specific intracellular receptor, is an effective releaser of Ca2+ from intracellular storage sites in the endoplasmic reticulum. Thus, the hydrolysis of phosphatidylinositol 4,5-bisphosphate leads to activation of PKC and promotes an increase of cytoplasmic Ca2+. As shown in Figure 42–4, the activation of G proteins can also have a direct action on Ca2+ channels. The resulting elevations of cytosolic Ca2+ activate Ca2+-calmodulin-dependent kinases and many other Ca2-calmodulin-dependent enzymes.
FIGURE 42–6 Certain hormone-receptor interactions result in the activation of phospholipase C (PLC). PLC activation appears to involve a specific G protein, which also may activate a calcium channel. Phospholipase C generates inositol trisphosphate (IP3), which liberates stored intracellular Ca2+, and diacylglycerol (DAG), a potent activator of protein kinase C (PKC). In this scheme, the activated PKC phosphorylates specific substrates, which then alter physiologic processes. Likewise, the Ca2+-calmodulin complex can activate specific kinases, two of which are shown here. These actions result in phosphorylation of substrates, and this leads to altered physiologic responses. This figure also shows that Ca2+ can enter cells through voltage- or ligand-gated Ca2+ channels. The intracellular Ca2+ is also regulated through storage and release by the mitochondria and endoplasmic reticulum. (Courtesy of JH Exton.)
FIGURE 42–7 Phospholipase C cleaves PIP2 into diacylglycerol and inositol trisphosphate. R1 generally is stearate, and R2 is usually arachidonate. IP3 can be dephosphorylated (to the inactive I-1,4-P2) or phosphorylated (to the potentially active I-1,3,4,5-P4).
Steroidogenic agents—including ACTH and cAMP in the adrenal cortex; angiotensin II, K+, serotonin, ACTH, and cAMP in the zona glomerulosa of the adrenal; LH in the ovary; and LH and cAMP in the Leydig cells of the testes—have been associated with increased amounts of phosphatidic acid, phosphatidylinositol, and polyphosphoinositides (see Chapter 15) in the respective target tissues. Several other examples could be cited.
The roles that Ca2+ and polyphosphoinositide breakdown products might play in hormone action are presented in Figure 42–6. In this scheme, the activated protein kinase C can phosphorylate specific substrates, which then alter physiologic processes. Likewise, the Ca2+-calmodulin complex can activate specific kinases. These then modify substrates and thereby alter physiologic responses.
Some Hormones Act Through a Protein Kinase Cascade
Single protein kinases such as PKA, PKC, and Ca2+-calmodulin (CaM)-kinases, which result in the phosphorylation of serine and threonine residues in target proteins, play a very important role in hormone action. The discovery that the EGF receptor contains an intrinsic tyrosine kinase activity that is activated by the binding of the ligand EGF was an important breakthrough. The insulin and IGF-I receptors also contain intrinsic ligand-activated tyrosine kinase activity. Several receptors—generally those involved in binding ligands involved in growth control, differentiation, and the inflammatory response—either have intrinsic tyrosine kinase activity or are associated with proteins that are tyrosine kinases. Another distinguishing feature of this class of hormone action is that these kinases preferentially phosphorylate tyrosine residues, and tyrosine phosphorylation is infrequent (<0.03% of total amino acid phosphorylation) in mammalian cells. A third distinguishing feature is that the ligand-receptor interaction that results in a tyrosine phosphorylation event initiates a cascade that may involve several protein kinases, phosphatases, and other regulatory proteins.
Insulin Transmits Signals by Several Kinase Cascades
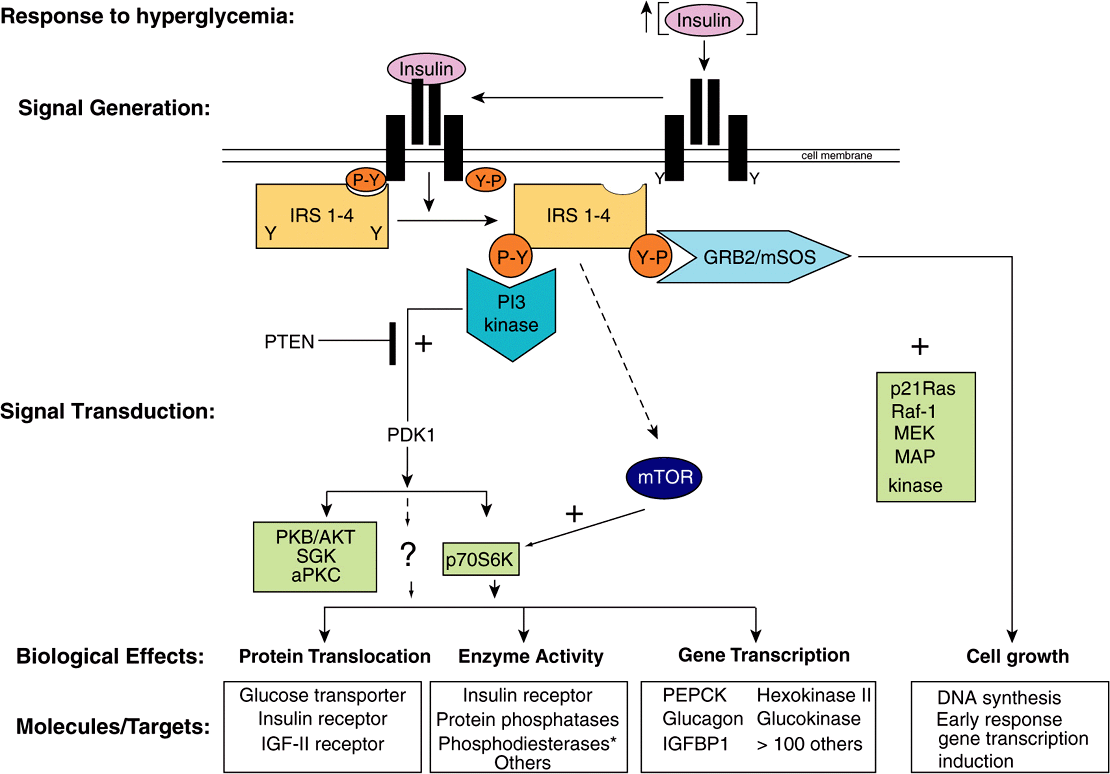
The insulin, epidermal growth factor (EGF), and IGF-I receptors have intrinsic protein tyrosine kinase activities located in their cytoplasmic domains. These activities are stimulated when their ligands bind to the cognate receptor. The receptors are then autophosphorylated on tyrosine residues, and this initiates a complex series of events (summarized in simplified fashion in Figure 42–8). The phosphorylated insulin receptor next phosphorylates insulin receptor substrates (there are at least four of these molecules, called IRS 1-4) on tyrosine residues. Phosphorylated IRS binds to the Src homology 2 (SH2) domains of a variety of proteins that are directly involved in mediating different effects of insulin. One of these proteins, PI-3 kinase, links insulin receptor activation to insulin action through activation of a number of molecules, including the kinase PDK1 (phosphoinositide-dependent kinase-1). This enzyme propagates the signal through several other kinases, including PKB (also known as AKT), SKG, and aPKC (see legend to Figure 42–8 for definitions and expanded abbreviations). An alternative pathway downstream from PDK1 involves p70S6K and perhaps other as yet unidentified kinases. A second major pathway involves mTOR. This enzyme is directly regulated by amino acid levels and insulin and is essential for p70S6K activity. This pathway provides a distinction between the PKB and p70S6K branches downstream from PKD1. These pathways are involved in protein translocation, enzyme activity, and the regulation, by insulin, of genes involved in metabolism (Figure 42–8). Another SH2 domain-containing protein is GRB2, which binds to IRS-1 and links tyrosine phosphorylation to several proteins, the result of which is activation of a cascade of threonine and serine kinases. A pathway showing how this insulin-receptor interaction activates the mitogen-activated protein (MAP) kinase pathway and the anabolic effects of insulin is illustrated in Figure 42–8. The exact roles of many of these docking proteins, kinases, and phosphatases remain to be established.
FIGURE 42–8 Insulin signaling pathways. The insulin signaling pathways provide an excellent example of the “recognition → hormone release → signal generation → effects” paradigm outlined in Figure 42–1. Insulin is released into the bloodstream from pancreatic β-cells in response to hyperglycemia. Binding of insulin to a target cell-specific plasma membrane heterotetrameric insulin receptor (IR) results in a cascade of intracellular events. First, the intrinsic tyrosine kinase activity of the insulin receptor is activated, and marks the initial event. Receptor activation results in increased tyrosine phosphorylation (conversion of specific Y residues → Y-P) within the receptor. One or more of the insulin receptor substrate (IRS) molecules (IRS 1-4) then bind to the tyrosine-phosphorylated receptor and themselves are specifically tyrosine phosphorylated. IRS proteins interact with the activated IR via N-terminal PH (plectsrin homology) and PTB (phosphotyrosine binding) domains. IR-docked IRS proteins are tyrosine phosphorylated and the resulting P-Y-residues form the docking sites for several additional signaling proteins (ie, PI-3 kinase, GRB2, and mTOR). GRB2 and PI3K bind to IRS P-Y residues via their SH (Src Homology) domains, Binding to IRS-Y-P residues leads to activation of the activity of many intracellular signaling molecules such as GTPases, protein kinases, and lipid kinases, all of which play key roles in certain metabolic actions of insulin. The two best-described pathways are shown. In detail, phosphorylation of an IRS molecule (probably IRS-2) results in docking and activation of the lipid kinase, PI-3 kinase; PI-3K generates novel inositol lipids that act as “second messenger” molecules. These, in turn, activate PDK1 and then a variety of downstream signaling molecules, including protein kinase B (PKB/AKT), SGK, and aPKC. An alternative pathway involves the activation of p70S6K and perhaps other as yet unidentified kinases. Next, phosphorylation of IRS (probably IRS-1) results in docking of GRB2/mSOS and activation of the small GTPase, p21Ras, which initiates a protein kinase cascade that activates Raf-1, MEK, and the p42/p44 MAP kinase isoforms. These protein kinases are important in the regulation of proliferation and differentiation of many cell types. The mTOR pathway provides an alternative way of activating p70S6K and appears to be involved in nutrient signaling as well as insulin action. Each of these cascades may influence different physiologic processes, as shown. All of the phosphorylation events are reversible through the action of specific phosphatases. As an example, the lipid phosphatase PTEN dephosphorylates the product of the PI-3 kinase reaction, thereby antagonizing the pathway and terminating the signal. Representative effects of major actions of insulin are shown in each of the boxes. The asterisk after phosphodiesterase indicates that insulin indirectly affects the activity of many enzymes by activating phosphodiesterases and reducing intracellular cAMP levels. (aPKC, atypical protein kinase C; GRB2, growth factor receptor binding protein 2; IGFBP, insulin-like growth factor binding protein; IRS 1-4, insulin receptor substrate isoforms 1-4; MAP kinase, mitogen-activated protein kinase; MEK, MAP kinase kinase and ERK kinase; mSOS, mammalian son of sevenless; mTOR, mammalian target of rapamycin; p70S6K, p70 ribosomal protein S6 kinase; PDK1, phosphoinositide-dependent kinase; PI-3 kinase, phosphatidylinositol 3-kinase; PKB, protein kinase B; PTEN, phosphatase and tensin homolog deleted on chromosome 10; SGK, serum and glucocorticoid-regulated kinase.)
The Jak/STAT Pathway is Used by Hormones and Cytokines
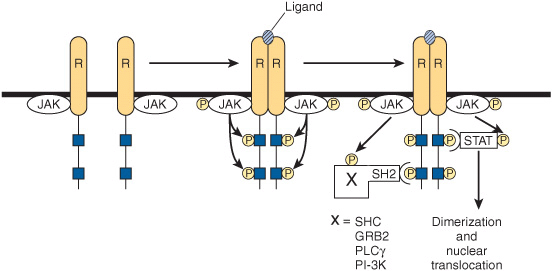
Tyrosine kinase activation can also initiate a phosphorylation and dephosphorylation cascade that involves the action of several other protein kinases and the counterbalancing actions of phosphatases. Two mechanisms are employed to initiate this cascade. Some hormones, such as growth hormone, prolactin, erythropoietin, and the cytokines, initiate their action by activating a tyrosine kinase, but this activity is not an integral part of the hormone receptor. The hormone-receptor interaction promotes binding and activation of cytoplasmic protein tyrosine kinases, such as Tyk-2, Jakl, or Jak2.
These kinases phosphorylate one or more cytoplasmic proteins, which then associate with other docking proteins through binding to SH2 domains. One such interaction results in the activation of a family of cytosolic proteins called signal transducers and activators of transcription (STATs). The phosphorylated STAT protein dimerizes and translocates into the nucleus, binds to a specific DNA element such as the interferon response element (IRE), and activates transcription. This is illustrated in Figure 42–9. Other SH2 docking events may result in the activation of PI-3 kinase, the MAP kinase pathway (through SHC or GRB2), or G protein-mediated activation of phospholipase C (PLCγ) with the attendant production of diacylglycerol and activation of protein kinase C. It is apparent that there is a potential for “cross-talk” when different hormones activate these various signal transduction pathways.
FIGURE 42–9 Initiation of signal transduction by receptors linked to Jak kinases. The receptors (R) that bind prolactin, growth hormone, interferons, and cytokines lack endogenous tyrosine kinase. Upon ligand binding, these receptors dimerize and an associated protein (Jak1, Jak2, or TYK) is phosphorylated. Jak-P, an active kinase, phosphorylates the receptor on tyrosine residues. The STAT proteins associate with the phosphorylated receptor and then are themselves phosphorylated by Jak-P. The phosphorylated STAT protein, STAT ![]() dimerizes, translocates to the nucleus, binds to specific DNA elements, and regulates transcription. The phosphotyrosine residues of the receptor also bind to several SH2 domain-containing proteins (X-SH2). This results in activation of the MAP kinase pathway (through SHC or GRB2), PLCγ, or PI-3 kinase.
dimerizes, translocates to the nucleus, binds to specific DNA elements, and regulates transcription. The phosphotyrosine residues of the receptor also bind to several SH2 domain-containing proteins (X-SH2). This results in activation of the MAP kinase pathway (through SHC or GRB2), PLCγ, or PI-3 kinase.
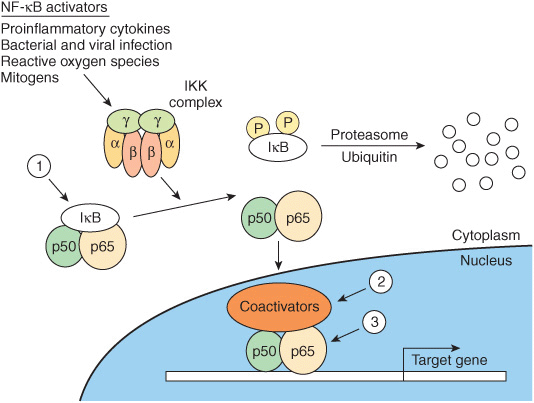
The NF-κB Pathway Is Regulated by Glucocorticoids
The transcription factor NF-κβ is a heterodimeric complex typically composed of two subunits termed p50 and p65 (Figure 42–10). Normally, NF-κβ is kept sequestered in the cytoplasm in a transcriptionally inactive form by members of the Inhibitor of NF-κβ (IκB) family. Extracellular stimuli such as proinflammatory cytokines, reactive oxygen species, and mitogens lead to activation of the IκB kinase complex, IKK, which is a heterohexameric structure consisting of α, β, and γ subunits. IKK phosphorylates IκB on two serine residues, and this targets IκB for ubiquitination and subsequent degradation by the proteasome. Following IκB degradation, free NF-κB translocates to the nucleus, where it binds to a number of gene promoters and activates transcription, particularly of genes involved in the inflammatory response. Transcriptional regulation by NF-κB is mediated by a variety of coactivators such as CREB binding protein (CBP), as described below (see Figure 42–13).
FIGURE 42–10 Regulation of the NF-κβ pathway. NF-κβ consists of two subunits, p50 and p65, which when present in the nucleus regulate transcription of the multitude of genes important for the inflammatory response. NF-κB is restricted from entering the nucleus by IκB, an inhibitor of NF-κB. IκB binds to—and masks—the nuclear localization signal of NF-κB. This cytoplasmic protein is phosphorylated by an IKK complex which is activated by cytokines, reactive oxygen species, and mitogens. Phosphorylated IκB can be ubiquitinylated and degraded, thus releasing its hold on NF-κB. Glucocorticoids, potent anti-inflammatory agents, are thought to affect at least three steps in this process (1, 2, 3), as described in the text.
Glucocorticoid hormones are therapeutically useful agents for the treatment of a variety of inflammatory and immune diseases. Their anti-inflammatory and immunomodulatory actions are explained in part by the inhibition of NF-κB and its subsequent actions. Evidence for three mechanisms for the inhibition of NF-κB by glucocorticoids has been presented: (1) glucocorticoids increase IκB mRNA, which leads to an increase of IκB protein and more efficient sequestration of NF-κB in the cytoplasm. (2) The glucocorticoid receptor competes with NF-κB for binding to coactivators. (3) The glucocorticoid receptor directly binds to the p65 subunit of NF-κB and inhibits its activation (Figure 42–10).
HORMONES CAN INFLUENCE SPECIFIC BIOLOGIC EFFECTS BY MODULATING TRANSCRIPTION
The signals generated as described above have to be translated into an action that allows the cell to effectively adapt to a challenge (Figure 42–1). Much of this adaptation is accomplished through alterations in the rates of transcription of specific genes. Many different observations have led to the current view of how hormones affect transcription. Some of these are as follows: (1) actively transcribed genes are in regions of “open” chromatin (experimentally defined as relative susceptibility to the enzyme DNase I), which allows for the access of transcription factors to DNA. (2) Genes have regulatory regions, and transcription factors bind to these to modulate the frequency of transcription initiation. (3) The hormone-receptor complex can be one of these transcription factors. The DNA sequence to which this binds is called a HRE (see Table 42-1 for examples). (4) Alternatively, other hormone-generated signals can modify the location, amount, or activity of transcription factors and thereby influence binding to the regulatory or response element. (5) Members of a large superfamily of nuclear receptors act with—or in a manner analogous to—the hormone receptors described above. (6) These nuclear receptors interact with another large group of coregulatory molecules to effect changes in the transcription of specific genes.
Several HREs Have Been Defined
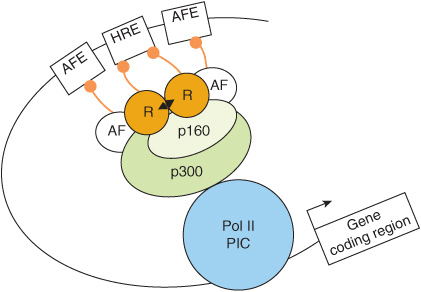
HREs resemble enhancer elements in that they are not strictly dependent on position and location or orientation. They generally are found within a few hundred nucleotides upstream (5’) of the transcription initiation site, but they may be located within the coding region of the gene, in introns. HREs were defined by the strategy illustrated in Figure 38–11. The consensus sequences illustrated in Table 42-1 were arrived at through analysis of many genes regulated by a given hormone using simple, heterologous reporter systems (see Figure 38–10). Although these simple HREs bind the hormone-receptor complex more avidly than surrounding DNA—or DNA from an unrelated source—and confer hormone responsiveness to a reporter gene, it soon became apparent that the regulatory circuitry of natural genes must be much more complicated. Glucocorticoids, progestins, mineralocorticoids, and androgens have vastly different physiologic actions. How could the specificity required for these effects be achieved through regulation of gene expression by the same HRE (Table 42-1)? Questions like this have led to experiments which have allowed for elaboration of a very complex model of transcription regulation. For example, the HRE must associate with other DNA elements (and associated binding proteins) to function optimally. The extensive sequence similarity noted between steroid hormone receptors, particularly in their DNA-binding domains (DBD), led to discovery of the nuclear receptor superfamily of proteins. These—and a large number of coregulator proteins—allow for a wide variety of DNA-protein and protein-protein interactions and the specificity necessary for highly regulated physiologic control. A schematic of such an assembly is illustrated in Figure 42–11.
FIGURE 42–11 The hormone response transcription unit. The hormone response transcription unit is an assembly of DNA elements and bound proteins that interact, through protein-protein interactions, with a number of coactivator or corepressor molecules. An essential component is the hormone response element that binds the ligand ![]() -bound receptor (R). Also important are the accessory factor elements (AFEs) with bound transcription factors. More than two dozen of these accessory factors (AFs), which are often members of the nuclear receptor superfamily, have been linked to hormone effects on transcription. The AFs can interact with each other, with the liganded nuclear receptors, or with coregulators. These components communicate with the basal transcription machinery, forming the Polymerase II PIC (ie, RNAP II and GTFs) through a coregulator complex that can consist of one or more members of the p160, corepressor, mediator-related, or CBP/p300 families (see Table 42-6). Recall (Chapters 36, 38) that many of the transcription coregulators carry intrinsic enzymatic activities, which covalently modify the DNA, transcription proteins, and the histones present in the nucleosomes (not shown here) in and around the enhancer (HRE, AFE) and promoter. Collectively the hormone, hormone receptor, chromatin, DNA and transcription machinery integrate and process hormone signals to regulate transcription in a physiological fashion.
-bound receptor (R). Also important are the accessory factor elements (AFEs) with bound transcription factors. More than two dozen of these accessory factors (AFs), which are often members of the nuclear receptor superfamily, have been linked to hormone effects on transcription. The AFs can interact with each other, with the liganded nuclear receptors, or with coregulators. These components communicate with the basal transcription machinery, forming the Polymerase II PIC (ie, RNAP II and GTFs) through a coregulator complex that can consist of one or more members of the p160, corepressor, mediator-related, or CBP/p300 families (see Table 42-6). Recall (Chapters 36, 38) that many of the transcription coregulators carry intrinsic enzymatic activities, which covalently modify the DNA, transcription proteins, and the histones present in the nucleosomes (not shown here) in and around the enhancer (HRE, AFE) and promoter. Collectively the hormone, hormone receptor, chromatin, DNA and transcription machinery integrate and process hormone signals to regulate transcription in a physiological fashion.
There Is a Large Family of Nuclear Receptor Proteins
The nuclear receptor superfamily consists of a diverse set of transcription factors that were discovered because of a sequence similarity in their DBDs. This family, now with >50 members, includes the nuclear hormone receptors discussed above, a number of other receptors whose ligands were discovered after the receptors were identified, and many putative or orphan receptors for which a ligand has yet to be discovered.
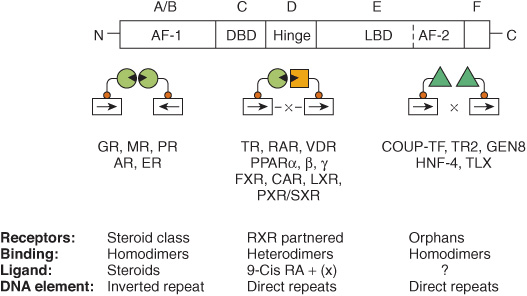
These nuclear receptors have several common structural features (Figure 42–12). All have a centrally located DBD that allows the receptor to bind with high affinity to a response element. The DBD contains two zinc finger binding motifs (see Figure 38–14) that direct binding either as homodimers, as heterodimers (usually with a retinoid X receptor [RXR] partner), or as monomers. The target response element consists of one or two DNA half-site consensus sequences arranged as an inverted or direct repeat. The spacing between the latter helps determine binding specificity. Thus, in general, a direct repeat with three, four, or five nucleotide spacer regions specifies the binding of the vitamin D, thyroid, and retinoic acid receptors, respectively, to the same consensus response element (Table 42-1). A multifunctional ligand-binding domain (LBD) is located in the carboxyl terminal half of the receptor. The LBD binds hormones or metabolites with selectivity and thus specifies a particular biologic response. The LBD also contains domains that mediate the binding of heat shock proteins, dimerization, nuclear localization, and transactivation. The latter function is facilitated by the carboxyl terminal transcription activation function (AF-2 domain), which forms a surface required for the interaction with coactivators. A highly variable hinge region separates the DBD from the LBD. This region provides flexibility to the receptor, so it can assume different DNA-binding conformations. Finally, there is a highly variable amino terminal region that contains another transactivation domain referred to as AF-1. The AF-1 domain likely provides for distinct physiologic functions through the binding of different coregulator proteins. This region of the receptor, through the use of different promoters, alternative splice sites, and multiple translation initiation sites, provides for receptor isoforms that share DBD and LBD identity but exert different physiologic responses because of the association of various coregulators with this variable amino terminal AF-1 domain.
FIGURE 42–12 The nuclear receptor superfamily. Members of this family are divided into six structural domains (A-F). Domain A/B is also called AF-1, or the modulator region, because it is involved in activating transcription. The C domain consists of the DNA-binding domain (DBD). The D region contains the hinge, which provides flexibility between the DBD and the ligand-binding domain (LBD, region E). The C terminal part of region E contains AF-2, another domain important for activation of transcription. The F region is poorly defined. The functions of these domains are discussed in more detail in the text. Receptors with known ligands, such as the steroid hormones, bind as homodimers on inverted repeat half-sites. Other receptors form heterodimers with the partner RXR on direct repeat elements. There can be nucleotide spacers of one to five bases between these direct repeats (DR1-5). Another class of receptors for which ligands have not been definitively determined (orphan receptors) bind as homodimers to direct repeats and occasionally as monomers to a single half-site.
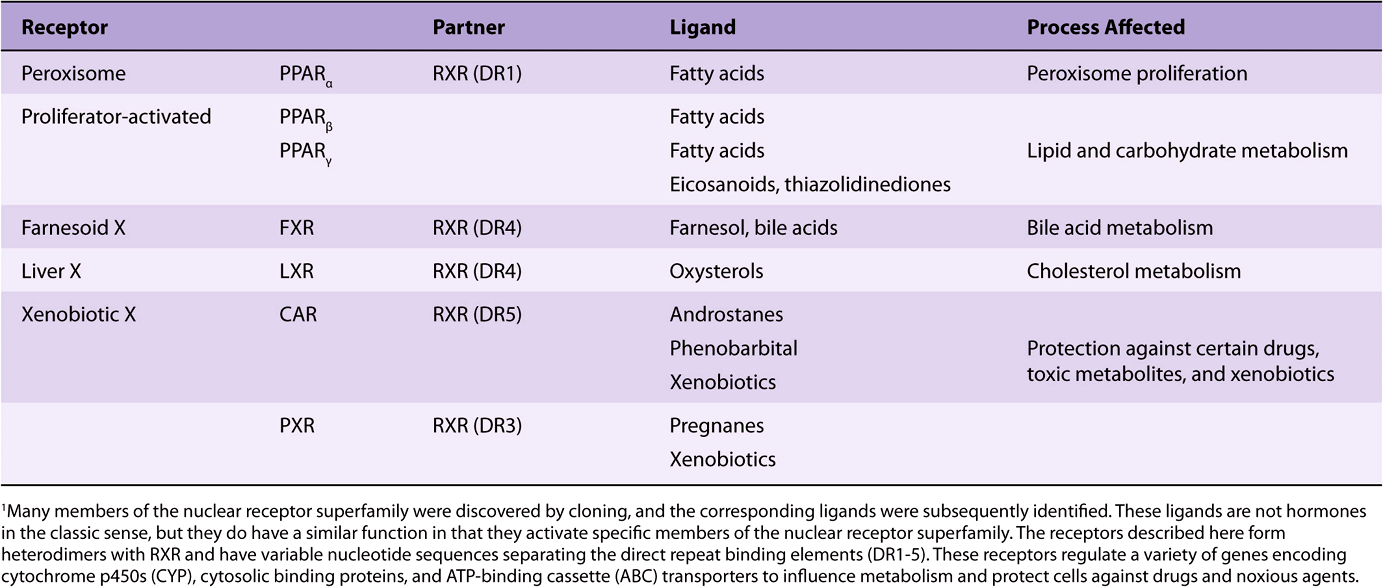
It is possible to sort this large number of receptors into groups in a variety of ways. Here, they are discussed according to the way they bind to their respective DNA elements (Figure 42–12). Classic hormone receptors for glucocorticoids (GR), mineralocorticoids (MR), estrogens (ER), androgens (AR), and progestins (PR) bind as homodimers to inverted repeat sequences. Other hormone receptors such as thyroid (TR), retinoic acid (RAR), and vitamin D (VDR) and receptors that bind various metabolite ligands such as PPAR α, β, and γ, FXR, LXR, PXR/SXR, and CAR bind as heterodimers, with retinoid X receptor (RXR) as a partner, to direct repeat sequences (see Figure 42–12 and Table 42-5). Another group of orphan receptors that as yet have no known ligand bind as homodimers or monomers to direct repeat sequences.
TABLE 42–5 Nuclear Receptors with Special Ligands1
As illustrated in Table 42-5, the discovery of the nuclear receptor superfamily has led to an important understanding of how a variety of metabolites and xenobiotics regulate gene expression and thus the metabolism, detoxification, and elimination of normal body products and exogenous agents such as pharmaceuticals. Not surprisingly, this area is a fertile field for investigation of new therapeutic interventions.
A Large Number of Nuclear Receptor Coregulators Also Participate in Regulating Transcription
Chromatin remodeling (histone modifications, DNA methylation), transcription factor modification by various enzyme activities, and the communication between the nuclear receptors and the basal transcription apparatus are accomplished by protein-protein interactions with one or more of a class of coregulator molecules. The number of these coregulator molecules now exceeds 100, not counting species variations and splice variants. The first of these to be described was the CREB-binding protein, CBP. CBP, through an amino terminal domain, binds to phosphorylated serine 137 of CREB and mediates transactivation in response to cAMP. It thus is described as a coactivator. CBP and its close relative, p300, interact directly or indirectly with a number of signaling molecules, including activator protein-1 (AP-1), STATs, nuclear receptors, and CREB (Figure 42–13). CBP/p300 also binds to the p160 family of coactivators described below and to a number of other proteins, including viral transcription factor Ela, the p90rsk protein kinase, and RNA helicase A. It is important to note, as mentioned above, that CBP/p300 also has intrinsic histone acetyltransferase (HAT) activity. Some of the many actions of CBP/p300, which appear to depend on intrinsic enzyme activities and its ability to serve as a scaffold for the binding of other proteins, are illustrated in Figure 42–11. Other coregulators serve similar functions.
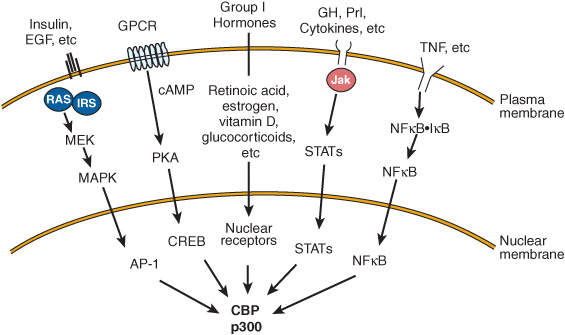
FIGURE 42–13 Several signal transduction pathways converge on CBP/p300. Many ligands that associate with membrane or nuclear receptors eventually converge on CBP/p300. Several different signal transduction pathways are employed. (EGF, epidermal growth factor; GH, growth hormone; Prl, prolactin; TNF, tumor necrosis factor; other abbreviations are expanded in the text.)
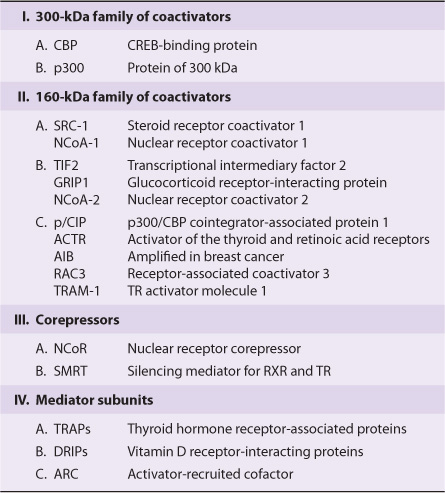
Several other families of coactivator molecules have been described. Members of the p160 family of coactivators, all of about 160 kDa, include (1) SRC-1 and NCoA-1; (2) GRIP 1, TIF2, and NCoA-2; and (3) p/CIP, ACTR, AIB1, RAC3, and TRAM-1 (Table 42-6). The different names for members within a subfamily often represent species variations or minor splice variants. There is about 35% amino acid identity between members of the different subfamilies. The p160 coactivators share several properties. They (1) bind nuclear receptors in an agonist- and AF-2 transactivation domain-dependent manner, (2) have a conserved amino terminal basic helix-loop-helix (bHLH) motif (see Chapter 38), (3) have a weak carboxyl terminal transactivation domain and a stronger amino terminal transactivation domain in a region that is required for the CBP/p160 interaction, (4) contain at least three of the LXXLL motifs required for protein–protein interaction with other coactivators, and (5) often have HAT activity. The role of HAT is particularly interesting, as mutations of the HAT domain disable many of these transcription factors. Current thinking holds that these HAT activities acetylate histones, which facilitates the remodeling of chromatin into a transcription-efficient environment. Histone acetylation/deacetylation thus plays a critical role in gene expression. Finally, it is important to note that other protein substrates for HAT-mediated acetylation, such as DNA binding transcription activators and other coregulators have been reported. Such nonhistone PTM events likely also factor importantly into the overall regulatory response.
TABLE 42–6 Some Mammalian Coregulator Proteins
A small number of proteins, including NCoR and SMRT, comprise the corepressor family. They function, at least in part, as described in Figure 42–2. Another family includes the TRAPs, DRIPs, and ARC (Table 42–6). These proteins represent subunits of the Mediator (Chapter 36) and range in size from 80 kDa to 240 kDa and are thought to link the nuclear receptor–coactivator complex to RNA polymerase II and the other components of the basal transcription apparatus.
The exact role of these coactivators is presently under intensive investigation. Many of these proteins have intrinsic enzymatic activities. This is particularly interesting in view of the fact that acetylation, phosphorylation, methylation, sumoylation, and ubiquitination—as well as proteolysis and cellular translocation—have been proposed to alter the activity of some of these coregulators and their targets.
It appears that certain combinations of coregulators—and thus different combinations of activators and inhibitors—are responsible for specific ligand-induced actions through various receptors. Furthermore, these interactions on a given promoter are dynamic. In some cases, complexes consisting of over 45 transcription factors have been observed on a single gene.
SUMMARY
![]() Hormones, cytokines, interleukins, and growth factors use a variety of signaling mechanisms to facilitate cellular adaptive responses.
Hormones, cytokines, interleukins, and growth factors use a variety of signaling mechanisms to facilitate cellular adaptive responses.
![]() The ligand-receptor complex serves as the initial signal for members of the nuclear receptor family.
The ligand-receptor complex serves as the initial signal for members of the nuclear receptor family.
![]() Class II peptide/protein and catecholamine hormones, which bind to cell surface receptors, generate a variety of intracellular signals. These include cAMP, cGMP, Ca2+, phosphatidylinositides, and protein kinase cascades.
Class II peptide/protein and catecholamine hormones, which bind to cell surface receptors, generate a variety of intracellular signals. These include cAMP, cGMP, Ca2+, phosphatidylinositides, and protein kinase cascades.
![]() Many hormone responses are accomplished through alterations in the rate of transcription of specific genes.
Many hormone responses are accomplished through alterations in the rate of transcription of specific genes.
![]() The nuclear receptor superfamily of proteins plays a central role in the regulation of gene transcription.
The nuclear receptor superfamily of proteins plays a central role in the regulation of gene transcription.
![]() Nuclear receptors, which may have hormones, metabolites, or drugs as ligands, bind to specific DNA elements as homodimers or as heterodimers with RXR. Some—orphan receptors—have no known ligand but bind DNA and influence transcription.
Nuclear receptors, which may have hormones, metabolites, or drugs as ligands, bind to specific DNA elements as homodimers or as heterodimers with RXR. Some—orphan receptors—have no known ligand but bind DNA and influence transcription.
![]() Another large family of coregulator proteins remodel chromatin, modify other transcription factors, and bridge the nuclear receptors to the basal transcription apparatus.
Another large family of coregulator proteins remodel chromatin, modify other transcription factors, and bridge the nuclear receptors to the basal transcription apparatus.
REFERENCES
Arvanitakis L, Geras-Raaka E, Gershengorn MC: Constitutively signaling G-protein-coupled receptors and human disease. Trends Endocrinol Metab 1998;9:27
Aylon Y, Oren M: Living with p53, Dying of p53. Cell 2007;130:597.
Beene DL, Scott JD: A-kinase anchoring proteins take shape. Current Opinion in Cell Biol 2007;19:192.
Brummer T, Schmitz-Perffer C, Daly RJ: Docking proteins. FEBS Journal 2010;277:4356-4369.
Cheung E, Kraus WL: Genomic Analyses of Hormone Signaling and Gene Regulation. Annu Rev Physiol 2010;72:191-218.
Darnell JE Jr, Kerr IM, Stark GR: Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science 1994;264:1415.
Fantl WJ, Johnson DE, Williams LT: Signalling by receptor tyrosine kinases. Annu Rev Biochem 1993;62:453.
Hanoune J, Defer N: Regulation and role of adenylyl cyclase isoforms. Annu Rev Pharmacol Toxicol 2001;41:145.
Jaken S: Protein kinase C isozymes and substrates. Curr Opin Cell Biol 1996;8:168.
Lee C-H, Olson P, Evans RM: Mini-review: Lipid metabolism, metabolic diseases and peroxisome proliferators-activated receptor. Endocrinology 2003;144:2201.
Métivier R, Gallais R, Tiffoche C, et al: Cyclical DNA methylation of a transcriptionally active promter. Nature 2008;452:45.
Métivier R, Reid G, Gannon F: Transcription in four dimensions: nuclear receptor-directed initiation of gene expression. EMBO Journal 2006;7:161.
Montminy M: Transcriptional regulation by cyclic AMP. Annu Rev Biochem 1997;66:807
Morris AJ, Malbon CC: Physiological regulation of G protein-linked signaling. Physiol Rev 1999;79:1373.
O’Malley B: Coregulators: from whence came these “master genes.” Mol Endocrinology 2007;21:1009.
Rosenfeld MG, Lunyak VV, Glass CK: Sensors and signals: a coactivator/corepressor/epigenetic code for integrating signal-dependent programs of transcriptional response. Genes and Dev 2006;20:1405.
Sonoda J, Pei L, Evans RM: Nuclear receptors: decoding metabolic disease. Fed of European Biochem Soc 2007;582:2.
Tang X, Tang G, Ozcan S: Role of microRNAs in diabetes. Biochim Biophys Acta 2008;1779:697.
Walton KM, Dixon JE: Protein tyrosine phosphatases. Annu Rev Biochem 1993;62:101.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree