Histiocytic Sarcoma
Roberto N. Miranda, MD
Key Facts
Terminology
Malignant neoplasm composed of mature histiocytes
Diagnosis based mainly on morphology and immunophenotype
Etiology/Pathogenesis
Transdifferentiation in subset of cases
B-cell lymphoma and HS are clonally related
Suggests that B cells can switch phenotype to histiocytic lineage
Clinical Issues
HS often presents as painless solitary mass
Clinical course can be indolent or aggressive
Bone marrow involvement is unusual
Must exclude acute monocytic leukemia/monocytic sarcoma
Microscopic Pathology
Diffuse pattern
Large cells with abundant eosinophilic cytoplasm
Ancillary Tests
Immunophenotype
CD163(+), CD68 (KP1 and PGM1)(+), and lysozyme(+)
CD45(+), CD45RO(+), and HLA-DR(+)
CD4(+/-), CD15(+/-)
Ki-67(+): 5-50% (median: 15%)
Top Differential Diagnoses
Monocytic/myeloid sarcoma
Langerhans cell histiocytosis/sarcoma
Malignant histiocytic tumor, unclassified
TERMINOLOGY
Abbreviations
Histiocytic sarcoma (HS)
Synonyms
True histiocytic lymphoma
Extramedullary monocytic tumor
Malignant histiocytosis is historical term
Not a true synonym as this term encompassed a number of entities
Definitions
Malignant neoplasm composed of mature histiocytes
Diagnosis mainly based on morphology and immunophenotype
Tumor cells are positive for histiocyte-associated markers, such as CD68, CD163, and lysozyme
Tumor cells are negative or show minor reactivity for dendritic or follicular dendritic cell markers
Monocytic/histiocytic neoplasms associated with acute myeloid leukemia, myeloproliferative neoplasms, or myelodysplastic syndromes are excluded
Better considered as monocytic sarcoma
ETIOLOGY/PATHOGENESIS
Postulated Normal Cell Counterpart
Phagocytic histiocyte or macrophage derived from bone marrow monocytes
Etiology
Unknown
Pathogenesis
Histiocytic and monocytic tumors are closely related
Some cases arise as 2nd malignancy after chemotherapy
Concept of “Transdifferentiation”
Patient has both lymphoid and histiocytic tumors that are clonally related
In most patients, histiocytic tumors follow or are synchronous with lymphoid neoplasms
Rare histiocytic tumors precede lymphoid neoplasms
This occurrence suggests that mature lymphoid cells can switch phenotypes to histiocytic lineage
Process may require initial de-differentiation &/or subsequent re-differentiation
Examples in literature include
HS and follicular lymphoma
HS and splenic marginal zone lymphoma
HS and B-lymphoblastic leukemia/lymphoma
Interdigitating dendritic cell sarcoma (IDCS) and follicular lymphoma
IDCS and chronic lymphocytic leukemia/small lymphocytic lymphoma
Histiocytic neoplasms associated with follicular lymphoma share
t(14;18)(q32;q21)/IgH-BCL2 and IgH rearrangements
Suggests common clonal origin of follicular lymphoma and histiocytic neoplasms
Supports that lymphoid neoplasms can transform into histiocytic neoplasms
An example of lineage plasticity
This concept also may encompass subset of sporadic histiocytic or dendritic cell sarcomas that
Bear monotypic IgH gene rearrangements
Show IgH/BCL2 translocation also identified in histiocytes
Express B-cell transcription factor Oct-2 but are negative for CD20, CD79a, and PAX-5
Possible mechanisms explaining monoclonal IgH gene rearrangements in HS
Lineage infidelity of primitive cells, supported by association with germ cell tumors
Dual genotype of histiocytes, which rearrange B- or T-cell antigen receptor genes
Artifactual detection of pseudoclones by polymerase chain reaction (PCR)
False-positive clonal rearrangements detected when there are too few lymphocytes for analysis
Duplicate testing tends to eliminate this artifact
True small clonal response by nonneoplastic lymphocytes to presence of tumor
Analysis of microdissected histiocytes may help define origin of clonal gene rearrangements
There is experimental evidence that immature or committed B cells can give rise to
Macrophages, natural killer cells, and T cells
CLINICAL ISSUES
Epidemiology
Incidence
HS is rare, with only a few cases reported
Most cases of “malignant histiocytosis” were described before immunohistochemical or molecular studies
Most of these cases are now recognized as other neoplasms
Most are diffuse large B-cell lymphoma and anaplastic large cell lymphoma
Cases diagnosed as “histiocytic lymphoma” in Rappaport classification are, in fact, large cell lymphoma
Age
Wide age range: 1-89 years
Median age: 51 years
Most cases occur in adults
Gender
Male to female ratio is 1.2 to 1
Site
Most cases arise in extranodal sites
Most common: Gastrointestinal tract, soft tissue, skin, spleen, and liver
Lymphadenopathy is less common
Presentation
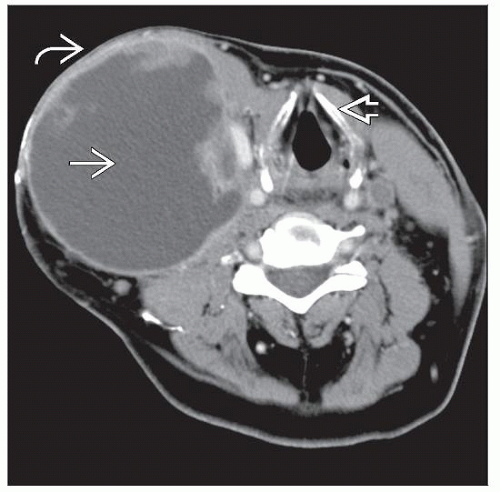
Usually presents as painless solitary mass
Lesions usually present for < 1 year
Most patients have stage I disease
Systemic symptoms, such as fever and weight loss, in subset of cases
Soft tissue masses may reach up to 12 cm in diameter
Skin manifestations are variable
Rash is common
Solitary or numerous lesions
Intestinal involvement may lead to abdominal pain, obstruction, or hematochezia
Bone marrow (BM) involvement is rare
Association with diffuse BM involvement is better considered as acute monocytic leukemia
Subset of cases with patchy BM involvement are considered as HS
Laboratory Tests
There are no diagnostic studies available
Pancytopenia or 1 or more cytopenias in some patients
Natural History
Clinical course can be indolent or aggressive
Treatment
Patients reported have not been uniformly treated
Surgical excision with wide margins was attempted when feasible
Combined chemotherapy and radiation therapy in subset of patients
Chemotherapy regimens used have been variable
CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone)
Regimens designed to treat acute leukemia
Prognosis
HS is often aggressive with poor response to therapy
60-80% of patients may die of progressive disease
Worst prognosis in patients with high-stage disease
Patients with clinically localized disease and small primary tumors have more favorable long-term outcome
Recurrences in subset of these patients (˜ 20%)
MACROSCOPIC FEATURES
General Features
Solitary mass is most common
Infiltrative margins
Median size: 7 cm (range: 1.8-12 cm)
MICROSCOPIC PATHOLOGY
Histologic Features
Focal or diffuse effacement of nodal or extranodal architecture
Focal nodal involvement is often paracortical
Sinusoidal distribution can occur but is uncommon
Soft tissue involvement displays infiltrative borders
Necrosis is frequent, and its extent is variable
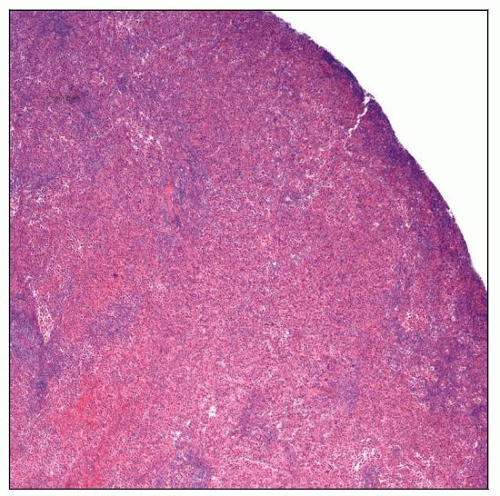
Neoplastic cells are large, noncohesive, and round to oval
Cells are usually > 20 µm in largest dimension
Abundant cytoplasm, usually eosinophilic
Spindle cells can be present focally
Hemophagocytosis by neoplastic cells can be present
Cytoplasmic vacuoles or xanthomatous appearance can be noted in some cases
Emperipolesis can be present focally
Mitotic figures are conspicuous but variable in number
Nuclei are large; central or eccentric location
Round to oval, or frequently with irregular folds and pleomorphic
Chromatin is fine, and nucleoli may be prominent
Few giant multinucleated cells are common
Usually prominent inflammatory background
Small lymphocytes, plasma cells, neutrophils, eosinophils, and benign histiocytes
When neutrophils are abundant, tumor can mimic inflammatory lesion
HS of central nervous system is notorious for heavy neutrophilic infiltrate
ANCILLARY TESTS
Immunohistochemistry
1 or more histiocytic or histiocyte-associated markers are positive
CD163(+), CD68 (KP1 and PGM1)(+), and lysozyme
CD68 and lysozyme: Cytoplasmic &/or Golgi/paranuclear pattern of staining
CD163: Membranous and cytoplasmic
Usually (> 90%) CD45(+), CD45RO(+), and HLA-DR(+)
CD4(+/-), CD15(+/- and dim)
S100(+/-): When positive, S100 is expressed in < 25% of neoplastic cells
Ki-67(+): 5-50% (median: 15%)
α-1-antitrypsin(+/-), α-1-antichymotrypsin(+/-)
Less sensitive and less specific; not widely used
CD1a(-), langerin/CD207(-)
Follicular dendritic cell markers(-)
CD21, CD23, CD35, and CNA.42
CD13(-), CD33(-), myeloperoxidase(-)
Pan-T-cell antigens(-)
B-cell markers(-)
Melanoma and carcinoma markers(-)
Flow Cytometry
Commonly positive
CD4, CD11c, CD45, CD45RO, CD68, CD163, and HLA-DR
Subset of cases positive
CD11b, CD13, CD15, CDw32, CD36, CD43, Mac387, and factor XIIIa
Cytogenetics
No characteristic findings
Isochromosome 12p in cases associated with germ cell tumors
Molecular Genetics
Usually negative for monoclonal T- and B-cell antigen receptor gene rearrangements
Variable proportion of cases show monoclonal IgH gene rearrangements by PCR
Histologic and immunophenotypic features are those of usual HS
Similar gene rearrangements or translocations can occur in HS and preceding B-cell lymphoma
Postulated to represent examples of “transdifferentiation”
Electron Microscopy
Cells show ample cytoplasm containing variable amounts of lysosomes and phagosomes
Negative for Birbeck granules, desmosomes, or cellular junctions
Enzyme Cytochemistry
Histiocytes are strongly positive for butyrate (nonspecific) esterase
Acid phosphatase(+/-)
Chloroacetate esterase(-)
Myeloperoxidase is usually negative, but it can be weakly positive in subset of cases
DIFFERENTIAL DIAGNOSIS
Monocytic/Myeloid Sarcoma
Neoplasm composed of monocytes
Usually associated with acute myeloid leukemia, myeloproliferative neoplasm, or myelodysplastic syndrome
Monocytic or myelomonocytic cell predominant represents approximately 40% of myeloid sarcomas
Small subset of these tumors is composed of large histiocytic rather than monocytic cells
Some authorities acknowledge 2 subsets of cases with histiocytic cytology
Commonly affected sites include lymph nodes, gastrointestinal tract, skin, and soft tissues
Histology and immunophenotype similar to HS
Less pleomorphic than HS
Ki-67 usually > 50%
Myeloperoxidase positive in subset of cases
Myelomonocytic marker MNDA(+) favors monocytic rather than histiocytic phenotype
Variably CD56(+)
Langerhans Cell Histiocytosis/Sarcoma
Cytologically bland cells with grooved or twisted nuclei
Pleomorphic cells in rare cases of Langerhans cell sarcoma
Eosinophils are commonly present
Immunophenotype
S100 protein(+), CD1a(+), and langerin/CD207(+)
Birbeck granules present by electron microscopy
Malignant Histiocytic Tumor, Unclassified
Malignant neoplasms with morphologic and ultrastructural features of HS
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree