11.1 INTRODUCTION TO HERPESVIRUSES
The word herpesvirus is derived from the Greek herpein, meaning to creep. More than 100 herpesviruses have been isolated and classified in the order Herpesvirales. Those found in mammals, birds, and reptiles constitute the family Herpesviridae, while other herpesviruses have been found in fish, amphibians, and a mollusk.
Herpesviruses are class I viruses, with genomes of dsDNA. A notable characteristic of herpesviruses is that, once they have infected a host, they commonly remain as persistent infections for the lifetime of the host. These infections are often latent infections, which can be reactivated from time to time, especially if the host becomes immunocompromised. Both primary and reactivated herpesvirus infections can either be asymptomatic or can result in disease of varying severity. The outcome depends on the interplay between the particular virus and its host, and especially on the immune status of the host.
11.2 THE HUMAN HERPESVIRUSES
There are eight herpesviruses known in man (Table 11.1), and most adults are persistently infected with most of them.
Table 11.1 Human herpesviruses
| Herpesvirus | Disease example(s) |
| Herpes simplex virus 1 | Cold sores |
| Herpes simplex virus 2 | Genital herpes |
| Varicella-zoster virus | Chickenpox, shingles |
| Epstein–Barr virus | Glandular fever, tumors |
| Human cytomegalovirus | Congenital defects |
| Human herpesvirus 6 | Exanthem subitum |
| Human herpesvirus 7 | Exanthem subitum |
| Kaposi’s sarcoma-associated herpesvirus | Kaposi’s sarcoma |
11.2.1 Herpes simplex viruses 1 and 2
Herpes simplex viruses 1 and 2 (HSV-1 and HSV-2) initially infect epithelial cells of the oral or genital mucosa, the skin or the cornea. The virus may spread to neurons in which it may be transported to their nuclei, where it may establish latent infections.
HSV-1 commonly infects via the lips or the nose between the ages of 6 and 18 months. A latent infection may be reactivated if, for example, the host becomes stressed or immunosuppressed. Reactivation results in the production of virions, which in about 20–40% of cases are transported within the neuron to the initial site of infection, where they cause productive infection in epithelial cells, resulting in a cold sore. Occasionally there may be serious complications such as encephalitis, especially in immunocompromised hosts.
HSV-2 is the usual causative agent of genital herpes, which is a sexually transmitted disease. In newborn babies infection can result in serious disease, with a mortality rate of about 54%.
Although the face and the genitals are the normal sites of infection for HSV-1 and HSV-2, respectively, there are increasing numbers of cases where HSV-1 infects the genitals and HSV-2 infects the face (Figure 11.1).
Figure 11.1 “Get your priorities right.”
Source: Haaheim, Pattison, and Whiteley (2002) A Practical Guide to Clinical Virology, 2nd edition. Reproduced by permission of John Wiley & Sons.
11.2.2 Varicella-zoster virus
Infection with varicella-zoster virus usually occurs in childhood and causes varicella (chickenpox), when the virus spreads through the blood to the skin, causing a rash. It may also spread to nerve cells, where it may establish a latent infection. The nerves most often affected are those in the face or the trunk, and these are the areas most commonly affected in zoster (shingles) when a latent infection is reactivated.
11.2.3 Epstein–Barr virus
Epstein–Barr virus (EBV) is transmitted in saliva. Epithelial cells are infected first then the infection spreads to B cells, which are the main host cell type for this virus. More than 90% of people become infected with EBV, usually during the first years of life, when infection results in few or no symptoms. In developed countries some individuals do not become infected until adolescence or adulthood. A proportion of these individuals develop infectious mononucleosis (glandular fever), commonly called “the kissing disease” by doctors. EBV is associated with a number of tumors in humans (Chapter 23).
11.2.4 Human cytomegalovirus
In the vast majority of infections with human cytomegalovirus, symptoms are either absent or they are mild. In a pregnant woman, however, the virus can infect the placenta and then the fetus, for whom the consequences may be serious. In one study it was estimated that 0.7% of children are born infected with the virus. In some of these there is evidence of virus-induced damage, including small brain size and enlargement of the liver and spleen; 0.5% of these congenitally infected babies die. Up to 20% of the survivors develop problems, include hearing loss and mental retardation.
Human cytomegalovirus can also cause severe disease (e.g. pneumonitis, hepatitis) in immunocompromised patients such as those with AIDS, those who have received treatment for cancer, and those who are immunosuppressed because they have received an organ transplant.
11.2.5 Human herpesvirus 6
There are two types of human herpesvirus 6, known as HHV-6A and HHV-6B. Infection of a child with the latter can cause a fever and the sudden appearance of a rash known as exanthem subitum.
11.2.6 Human herpesvirus 7
Human herpesvirus 7 was first isolated from a culture of CD4 T cells that developed a cytopathic effect; the cells were from a healthy person. The virus has been associated with some cases of exanthem subitum.
11.2.7 Kaposi’s sarcoma-associated herpesvirus
Kaposi’s sarcoma-associated herpesvirus was discovered in 1994 and is named after the tumor with which the virus is associated (Chapter 23).
11.3 THE HERPESVIRUS VIRION
Herpesviruses have relatively complex virions composed of a large number of protein species organized into three distinct structures: capsid, tegument, and envelope (Figure 11.2). The virus genome is a linear dsDNA molecule, which is about 125 kbp in the smallest herpesviruses and about 290 kbp in the largest herpesviruses. The DNA is housed in the capsid, which is icosahedral, and the capsid is surrounded by the tegument. Many of the envelope glycoprotein molecules are organized into spikes of various dimensions.
Figure 11.2 The herpesvirus virion.
Source: (b) and (c) are images of HSV-1, from Grünewald and Cyrklaff (2006) Current Opinion in Microbiology, 9, 437. Reproduced by permission of the authors and Elsevier Limited.
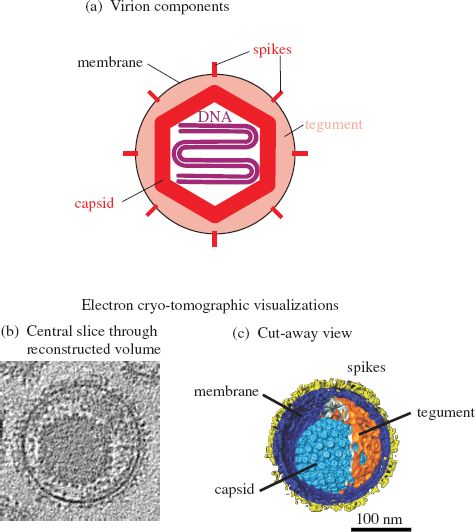
In the HSV-1 virion the capsid sits asymmetrically within the tegument (Figure 11.2(b) and (c)). The HSV-1 tegument contains at least 26 species of virus protein, some cell proteins, and some virus mRNA molecules. The envelope contains at least 16 protein species, most of which are glycoproteins forming 600–750 spikes.
The capsid is constructed from 162 capsomeres, 150 of which are hexons (Figure 11.3). At one of the vertices of the icosahedron there is a structure called the portal. There is evidence from electron microscopy of HSV-1 capsids treated with antibody specific for pUL6 that this protein forms the portal. The virus DNA passes through the portal when entering the procapsid during packaging, and it is likely that it also passes through the portal when leaving the capsid during the infection process. There is a penton at each of the other eleven vertices of the icosahedron.
Figure 11.3 The HSV-1 capsid. Reconstructed image from cryo-electron microscopy.
Source: Courtesy of Professor Wah Chiu, Baylor College of Medicine, Houston, TX. Reinterpretation of data from Zhou et al. (2000) Science, 288, 877, with permission of the American Association for the Advancement of Science.
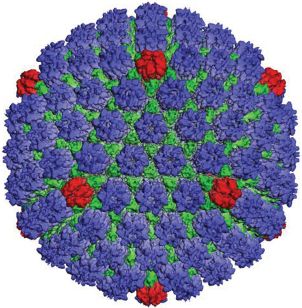
A number of schemes have evolved for the nomenclature of herpesvirus proteins, with the result that an individual protein may have two or more different names in the literature. Most of the structural proteins are commonly named VP (virus protein). In HSV-1 the most abundant protein in the tegument is VP16, while the pentons and hexons are composed of VP5; a penton is made from five molecules and a hexon from six molecules of VP5. Other proteins make up structures called triplexes, which connect the capsomeres. The envelope glycoproteins are each prefixed “g”, for example, gB, gC, and gD.
11.4 HSV-1 GENOME ORGANIZATION
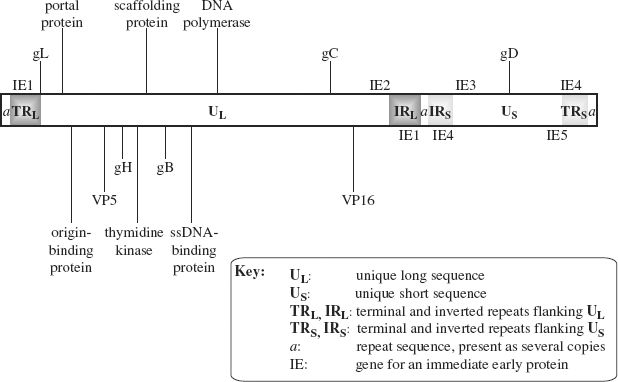
HSV-1 is one of the most studied herpesviruses; we shall look at its genome organization and then at its replication. The genome consists of two unique sequences each flanked by repeat sequences (Figure 11.4). The unique sequences are not of equal length: the longer is designated UL and the shorter is designated US. A sequence known as the a sequence is present at each end of the genome, and at the junction of the inverted repeat (IR) sequences. The a sequence plays an important role during replication when genomes are packaged into procapsids.
Figure 11.4 The HSV-1 genome.
The locations of some genes are indicated. Those shown above the genome are in ORFs read left to right; those shown below the genome are in ORFs read right to left.
The locations of the genes for the five immediate early (IE) proteins are indicated. Two of these genes are in repeat sequences, so there are two copies of these genes.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


