INTRODUCTION
The herpesvirus family contains several important human viral pathogens. Clinically, the herpesviruses exhibit a wide spectrum of diseases. Some have an extensive host-cell range, and others have a narrow host-cell range. The outstanding property of herpesviruses is their ability to establish lifelong persistent infections in their hosts and to undergo periodic reactivation. Their frequent reactivation in elderly and immunosuppressed patients causes serious health complications. Curiously, the reactivated infection may be clinically quite different from the disease caused by the primary infection. Herpesviruses possess a large number of genes, some of which have proved to be susceptible to antiviral chemotherapy.
The herpesviruses that commonly infect humans are numbered from human herpesvirus 1 (HHV-1) to HHV-8, but are commonly referred to by their individual virus names. In order, these are herpes simplex virus types 1 and 2 (HSV-1, HSV-2), varicella-zoster virus (VZV), cytomegalovirus (CMV), Epstein-Barr virus (EBV), herpesviruses 6 (HHV-6), herpesvirus 7 (HHV-7), and herpesvirus 8 (HHV-8, also known as Kaposi sarcoma-associated herpesvirus [KSHV]). Herpes B virus of monkeys can also infect humans. There are nearly 100 viruses of the herpes group that infect many different animal species.
PROPERTIES OF HERPESVIRUSES
Important properties of herpesviruses are summarized in Table 33-1.
| Virion: Spherical, 150–200 nm in diameter (icosahedral) |
| Genome: Double-stranded DNA, linear, 125–240 kbp, reiterated sequences |
| Proteins: More than 35 proteins in virion |
| Envelope: Contains viral glycoproteins, Fc receptors |
| Replication: Nucleus, bud from nuclear membrane |
| Outstanding characteristics: |
| Encode many enzymes |
| Establish latent infections |
| Persist indefinitely in infected hosts |
| Frequently reactivated in immunosuppressed hosts |
| Some cause cancer |
Herpesviruses are large viruses. Different members of the group share architectural details and are indistinguishable by electron microscopy. All herpesviruses have a core of double-stranded DNA, in the form of a toroid, surrounded by a protein coat that exhibits icosahedral symmetry and has 162 capsomeres. The nucleocapsid is surrounded by an envelope that is derived from the nuclear membrane of the infected cell and contains viral glycoprotein spikes about 8 nm long. An amorphous, sometimes asymmetric structure between the capsid and envelope is designated the tegument. The enveloped form measures 150–200 nm; the “naked” virion, 125 nm.
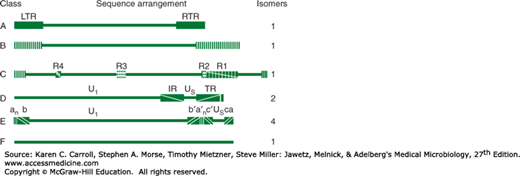
The double-stranded DNA genome (125–240 kbp) is linear. A striking feature of herpesvirus DNAs is their sequence arrangement (Figure 33-1). Herpesvirus genomes possess terminal and internal repeated sequences. Some members, such as the HSVs, undergo genome rearrangements, giving rise to different genome “isomers.” The base composition of herpesvirus DNAs varies from 31% to 75% (G + C). There is little DNA homology among different herpesviruses except for HSV-1 and HSV-2, which show 50% sequence homology, and human herpesviruses 6 and 7 (HHV-6 and HHV-7), which display limited (30–50%) sequence homology. Treatment with restriction endonucleases yields characteristically different cleavage patterns for herpesviruses and even for different strains of each type. This “fingerprinting” of strains allows epidemiologic tracing of a given strain.
FIGURE 33-1
Schematic diagram of sequence arrangements of herpesvirus DNAs. Genome classes A, B, C, D, E, and F are exemplified by channel catfish virus, herpesvirus saimiri, Epstein-Barr virus, varicella-zoster virus, herpes simplex viruses, and tupaia herpesvirus, respectively. Horizontal lines represent unique regions. Reiterated domains are shown as rectangles: left and right terminal repeats (LTR and RTR) for class A; repeats R1–R4 for internal repeats of class C; and internal and terminal repeats (IR and TR) of class D. In class B, terminal sequences are reiterated numerous times at both termini. The termini of class E consist of two elements. The terminal sequences (ab and ca) are inserted in an inverted orientation separating the unique sequences into long (Ul) and short (Us) domains. Genomes of class F have no terminal reiterations. The components of the genomes in classes D and E invert. In class D (varicella-zoster virus), the short component inverts relative to the long, and the DNA forms two populations (isomers) differing in the orientation of the short component. In class E (herpes simplex virus), both the short and long components can invert, and viral DNA consists of four isomers. (Reproduced with permission from Roizman B: Herpesviridae: A brief introduction. In Fields BN, Knipe DM [editors-in-chief]. Virology, 2nd ed. Raven Press, 1990, pp. 1787–1793.)
The herpesvirus genome is large and encodes at least 100 different proteins. Of these, more than 35 polypeptides are involved in the structure of the virus particle; at least 10 are part of the viral envelope. Herpesviruses encode an array of virus-specific enzymes involved in nucleic acid metabolism, DNA synthesis, gene expression, and protein regulation (DNA polymerase, helicase-primase, thymidine kinase, transcription factors, protein kinases). Many herpesvirus genes appear to be viral homologs of cellular genes.
Taxonomic classification of the numerous members of the herpesvirus family is complicated. A useful division into subfamilies is based on biologic properties of the agents (Table 33-2). Alphaherpesviruses are fast-growing, cytolytic viruses that tend to establish latent infections in neurons; HSV (genus Simplexvirus) and VZVs (genus Varicellovirus) are members. Betaherpesviruses are slow growing and may be cytomegalic (massive enlargements of infected cells) and become latent in secretory glands and kidneys; CMV is classified in the Cytomegalovirus genus. Also included here, in the genus Roseolovirus, are HHV-6 and HHV-7; by biologic criteria, they are similar to gammaherpesviruses because they infect lymphocytes (T lymphotropic), but molecular analyses of their genomes reveal that they are more closely related to the betaherpesviruses. Gammaherpesviruses, exemplified by EBV (genus Lymphocryptovirus), infect and become latent in lymphoid cells. HHV-8/KSHV is classified in the Rhadinovirus genus.
| Subfamily (“-herpesvirinae”) | Biologic Properties | Genus (“-virus”) | Examples | ||
|---|---|---|---|---|---|
| Growth Cycle and Cytopathology | Latent Infections | Official Name (“Human Herpesvirus”) | Common Name | ||
| Alpha | Short, cytolytic | Neurons | Simplex | 1 | Herpes simplex virus type 1 |
| 2 | Herpes simplex virus type 2 | ||||
| Varicello | 3 | Varicella-zoster virus | |||
| Beta | Long, cytomegalic | Glands, kidneys | Cytomegalo | 5 | Cytomegalovirus |
| Long, lymphoproliferative | Lymphoid tissue | Roseolo | 6 7 | Human herpesvirus 6 Human herpesvirus 7 | |
| Gamma | Variable, lymphoproliferative | Lymphoid tissue | Lymphocrypto Rhadino | 4 8 | Epstein-Barr virus Kaposi sarcoma-associated herpesvirus |
Many herpesviruses infect animals, the most notable being B virus (herpesvirus simiae or cercopithecine herpesvirus 1) in the Simplexvirus genus; herpesviruses saimiri and ateles of monkeys, both in genus Rhadinovirus; marmoset herpesvirus (genus Simplexvirus); and pseudorabies virus of pigs and infectious bovine rhinotracheitis virus of cattle, both in genus Varicellovirus.
There is little antigenic relatedness among members of the herpesvirus group. Only HSV-1 and HSV-2 share a significant number of common antigens. HHV-6 and HHV-7 exhibit a few cross-reacting epitopes.
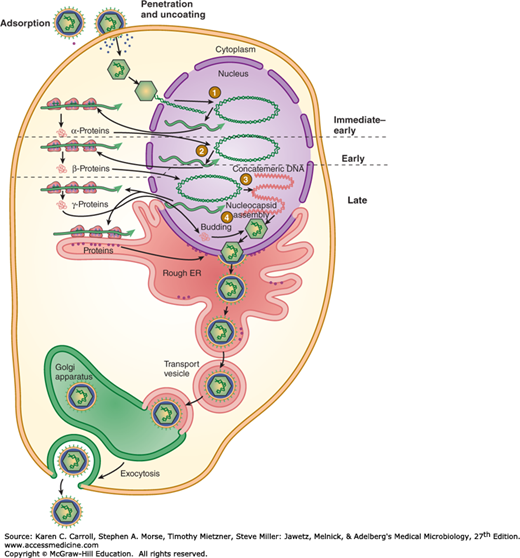
The replication cycle of HSV is summarized in Figure 33-2. The virus enters the cell by fusion with the cell membrane after binding to specific cellular receptors via envelope glycoproteins. Several herpesviruses bind to cell surface glycosaminoglycans, principally heparan sulfate. Virus attachment also involves binding to one of several coreceptors (eg, members of the immunoglobulin superfamily). After fusion, the capsid is transported through the cytoplasm to a nuclear pore, uncoating occurs, and the DNA becomes associated with the nucleus. The viral DNA forms a circle immediately upon release from the capsid. Expression of the viral genome is tightly regulated and sequentially ordered in a cascade fashion. VP16, a tegument protein, complexes with several cellular proteins and activates initial viral gene expression. Immediate-early genes are expressed, yielding “α” proteins. These proteins permit expression of the early set of genes, which are translated into “β” proteins. Viral DNA replication begins, and late transcripts are produced that give rise to “γ” proteins. More than 50 different proteins are synthesized in herpesvirus-infected cells. Many α and β proteins are enzymes or DNA-binding proteins; most of the γ proteins are structural components.
FIGURE 33-2
Replication cycle of herpes simplex virus. (1) Virus fuses with plasma membrane, and viral DNA is released from capsid at nuclear pore followed by circularization of genome and transcription of immediate-early genes. (2) α-Proteins, products of immediate-early genes, stimulate transcription of early genes. (3) β-Proteins, products of early genes, function in DNA replication, yielding concatemeric DNA. Late genes are transcribed. (4) γ-Proteins, products of late genes and consisting primarily of viral structural proteins, participate in virion assembly. Unit-length viral DNA is cleaved from concatemers and packaged into capsids. Enveloped viral particles accumulate in the endoplasmic reticulum (ER) and are transported from the cell. (Reproduced with permission from Willey JM, Sherwood LM, Woolverton CJ: Prescott, Harley, and Klein’s Microbiology, 7th ed. McGraw-Hill, 2008. © The McGraw-Hill Companies, Inc.)
Viral DNA is transcribed throughout the replicative cycle by cellular RNA polymerase II but with the participation of viral factors. Viral DNA is synthesized by a rolling-circle mechanism. Herpesviruses differ from other nuclear DNA viruses in that they encode a large number of enzymes involved in DNA synthesis. These enzymes have been good targets for development of antiviral drugs. Newly synthesized viral DNA is packaged into preformed empty nucleocapsids in the cell nucleus.
Maturation occurs by budding of nucleocapsids through the altered inner nuclear membrane. Enveloped virus particles are then transported by vesicular movement to the surface of the cell.
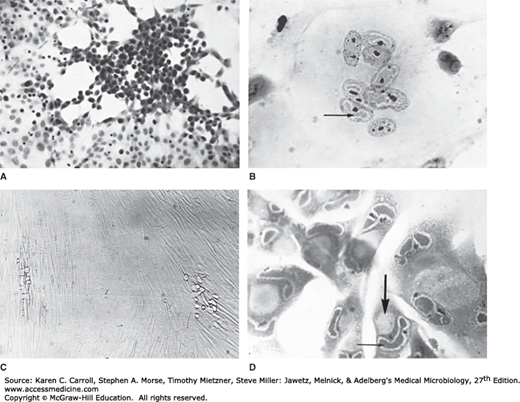
The length of the replication cycle varies from about 18 hours for HSV to more than 70 hours for CMV. Cells productively infected with herpesviruses are invariably killed. Host macromolecular synthesis is shut off early in infection; normal cellular DNA and protein synthesis virtually stop as viral replication begins. Cytopathic effects induced by human herpesviruses are quite distinct and can include intranuclear inclusion bodies (Figure 33-3).
FIGURE 33-3
Cytopathic effects induced by herpesviruses. A: Herpes simplex virus in HEp-2 cells (hematoxylin and eosin stain, 57×), with early focus of swollen, rounded cells. B: Varicella-zoster virus in human kidney cells (hematoxylin and eosin stain, 228×), with multinucleated giant cell containing acidophilic intranuclear inclusions (arrow). C: Cytomegalovirus in human fibroblasts (unstained, 35×) with two foci of slowly developing cytopathic effect. D: Cytomegalovirus in human fibroblasts (hematoxylin and eosin stain, 228×), showing giant cells with acidophilic inclusions in the nuclei (small arrow) and cytoplasm (large arrow), the latter being characteristically large and round. (Courtesy of I Jack; reproduced from White DO, Fenner FJ: Medical Virology, 3rd ed. Academic Press, 1986.)
The number of potential protein-coding open-reading frames in herpesvirus genomes ranges from about 70 to more than 200. In the case of HSV, about half the genes are not needed for growth in cultured cells. The other genes are probably required for viral survival in vivo in natural hosts.
Herpesviruses have been found to express multiple microRNAs, small (~22 nucleotides) single-stranded RNAs that function posttranscriptionally to regulate gene expression. These viral microRNAs are important in regulating cellular functions and entry into or exit from (or both) the latent phase of the virus life cycle, and provide attractive targets for novel antiviral therapy development.
A wide variety of diseases are associated with infection by herpesviruses. Primary infection and reactivated disease by a given virus may involve different cell types and present different clinical pictures.
HSV-1 and HSV-2 infect epithelial cells and establish latent infections in neurons. Type 1 is classically associated with oropharyngeal lesions and causes recurrent attacks of “fever blisters.” Type 2 primarily infects the genital mucosa and is mainly responsible for genital herpes, though the anatomical specificity of these viruses is diminishing. Both viruses can also cause neurologic disease. HSV-1 is the leading viral cause of sporadic encephalitis in the United States. Both types 1 and 2 can cause neonatal infections that are often severe.
VZV causes chickenpox (varicella) on primary infection and establishes latent infection in neurons. Upon reactivation, the virus causes herpes zoster (shingles). Adults who are infected for the first time with varicella-zoster virus can develop serious viral pneumonia.
CMV replicates in epithelial cells of the respiratory tract, salivary glands, and kidneys and persists in lymphocytes. It causes an infectious mononucleosis (heterophile antibody-negative). In newborns, disseminated cytomegalic inclusion disease may occur. CMV is an important cause of congenital defects, neonatal hearing loss, and mental retardation.
EBV replicates in epithelial cells of the oropharynx and parotid gland and establishes latent infections in lymphocytes. It causes infectious mononucleosis and can induce human lymphoproliferative disorders, especially in immunocompromised patients
HHV-6 infects T lymphocytes. It is typically acquired in early infancy and causes exanthem subitum (roseola infantum) as well as infections in immunocompromised patients. HHV-7, also a T-lymphotropic virus, has not yet been linked to any specific disease. HHV-8 is associated with the development of Kaposi sarcoma, a vascular tumor that is common in patients with AIDS.
Herpes B virus of macaque monkeys can infect humans upon exposure to live animals or tissue samples. Such infections are rare, but those that occur usually result in severe neurologic disease and are frequently fatal.
Human herpesviruses are frequently reactivated in the elderly and immunosuppressed patients (eg, transplant recipients, cancer patients) and may cause severe disease, such as pneumonia or lymphomas.
Herpesviruses have been linked with malignant diseases in humans and lower animals: EBV with Burkitt lymphoma of African children, with nasopharyngeal carcinoma, and with other lymphoproliferative disorders; KSHV with Kaposi sarcoma; Marek disease virus with a lymphoma of chickens; and a number of primate herpesviruses with reticulum cell sarcomas and lymphomas in monkeys.
HERPESVIRUS INFECTIONS IN HUMANS
HSV are extremely widespread in the human population. They exhibit a broad host range, being able to replicate in many types of cells and to infect many different animals. They grow rapidly and are highly cytolytic. The HSVs are responsible for a spectrum of diseases, ranging from gingivostomatitis to keratoconjunctivitis, encephalitis, genital disease, and infections of newborns. The HSVs establish latent infections in nerve cells and recurrences are common.
There are two distinct HSV, types 1 and 2 (HSV-1 and HSV-2) (Table 33-3). Their genomes are similar in organization and exhibit substantial sequence homology. However, they can be distinguished by sequence analysis or by restriction enzyme analysis of viral DNA. The two viruses cross-react serologically, but some unique proteins exist for each type. Classically, HSV-1 is spread by contact, usually involving infected saliva and HSV-2 is transmitted sexually or from a maternal genital infection to a newborn. However, these patterns are becoming less distinct, and both viruses can cause either presentation.
| Characteristics | HSV-1 | HSV-2 |
|---|---|---|
| Biochemical | ||
| Viral DNA base composition (G + C) (%) | 67 | 69 |
| Buoyant density of DNA (g/cm3) | 1.726 | 1.728 |
| Buoyant density of virions (g/cm3) | 1.271 | 1.267 |
| Homology between viral DNAs (%) | ~50 | ~50 |
| Biologic | ||
| Animal vectors or reservoirs | None | None |
| Typical site of latency | Trigeminal ganglia | Sacral ganglia |
| Epidemiologic | ||
| Age of primary infection | Young children | Young adults |
| Typical transmission | Contact (often saliva) | Sexual |
| Typical clinical associations | ||
| Primary infection: | ||
| Gingivostomatitis | + | − |
| Pharyngotonsillitis | + | − |
| Keratoconjunctivitis | + | − |
| Neonatal infections | ± | + |
| Recurrent infection: | ||
| Cold sores, fever blisters | + | − |
| Keratitis | + | − |
| Primary or recurrent infection: | ||
| Cutaneous herpes | ||
| Skin above the waist | + | ± |
| Skin below the waist | ± | + |
| Hands or arms | + | + |
| Herpetic whitlow | + | + |
| Eczema herpeticum | + | − |
| Genital herpes | ± | + |
| Herpes encephalitis | + | − |
| Herpes meningitis | ± | + |
The HSV growth cycle proceeds rapidly, requiring 8–16 hours for completion. The HSV genome is large (~150 kbp) and can encode at least 70 polypeptides; the functions of many of the proteins in replication or latency are not known. At least eight viral glycoproteins are among the viral late gene products. One (gD) is the most potent inducer of neutralizing antibodies. Glycoprotein C is a complement (C3b)-binding protein, and gE is an Fc receptor, binding to the Fc portion of immunoglobulin G (IgG). Glycoprotein G is type specific and allows for antigenic discrimination between HSV-1 (gG-1) and HSV-2 (gG-2).
Because HSV causes cytolytic infections, pathologic changes are due to necrosis of infected cells together with the inflammatory response. Lesions induced in the skin and mucous membranes by HSV-1 and HSV-2 are the same and resemble those of VZV. Changes induced by HSV are similar for primary and recurrent infections but vary in degree, reflecting the extent of viral cytopathology.
Characteristic histopathologic changes include ballooning of infected cells, production of Cowdry type A intranuclear inclusion bodies, margination of chromatin, and formation of multinucleated giant cells. Cell fusion provides an efficient method for cell-to-cell spread of HSV, even in the presence of neutralizing antibody.
HSV is transmitted by contact of a susceptible person with an individual excreting virus. The virus must encounter mucosal surfaces or broken skin for an infection to be initiated (unbroken skin is resistant). Viral replication occurs first at the site of infection. Virus then invades local nerve endings and is transported by retrograde axonal flow to dorsal root ganglia, where, after further replication, latency is established. Whereas oropharyngeal HSV infections result in latent infections in the trigeminal ganglia, genital HSV infections lead to latently infected sacral ganglia.
Primary HSV infections are usually mild; in fact, most are asymptomatic. Only rarely does systemic disease develop. Occasionally HSV can enter the central nervous system and cause meningitis or encephalitis. Widespread organ involvement can result when an immunocompromised host is not able to limit viral replication and viremia ensues.
Virus resides in latently infected ganglia in a nonreplicating state; only a very few viral genes are expressed. Viral persistence in latently infected ganglia lasts for the lifetime of the host. No virus can be recovered between recurrences at or near the usual site of recurrent lesions. Provocative stimuli can reactivate virus from the latent state, including axonal injury, fever, physical or emotional stress, and exposure to ultraviolet light. The virus follows axons back to the peripheral site, and replication proceeds at the skin or mucous membranes. Spontaneous reactivations occur despite HSV-specific humoral and cellular immunity in the host. However, this immunity limits local viral replication, so that recurrent infections are less extensive and less severe. Many recurrences are asymptomatic, reflected only by viral shedding in secretions. When symptomatic, episodes of recurrent HSV are most often manifested as cold sores (fever blisters) near the lip. More than 80% of the human population harbor HSV-1 in a latent form, but only a small portion experience recurrences. It is not known why some individuals have reactivations and others do not.
HSV-1 and HSV-2 may cause many clinical entities, and the infections may be primary or recurrent (see Table 33-3). Primary infections occur in persons without antibodies and in most individuals are clinically inapparent but result in antibody production and establishment of latent infections in sensory ganglia. Recurrent lesions are common.
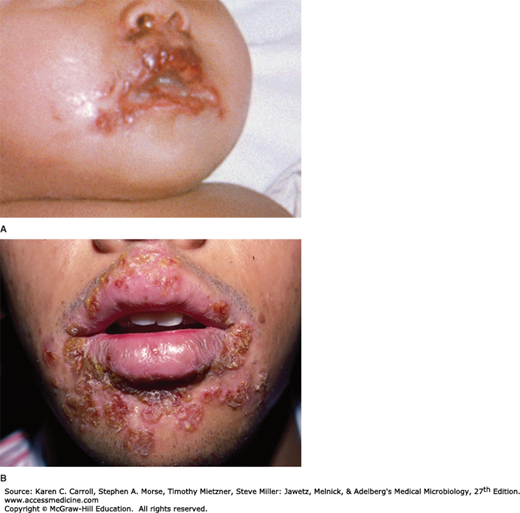
Primary HSV-1 infections are usually asymptomatic. Symptomatic disease occurs most frequently in small children (1–5 years of age) and involves the buccal and gingival mucosa of the mouth (Figure 33-4A). The incubation period is short (~3–5 days, with a range of 2–12 days), and clinical illness lasts 2–3 weeks. Symptoms include fever, sore throat, vesicular and ulcerative lesions, gingivostomatitis, and malaise. Gingivitis (swollen, tender gums) is the most striking and common lesion. Primary infections in adults commonly cause pharyngitis and tonsillitis. Localized lymphadenopathy may occur.
FIGURE 33-4
A: Primary herpes simplex gingivostomatitis. (Courtesy of JD Millar. Source: Centers for Disease Control and Prevention, Public Health Image Library, ID# 2902, 2008). B: Recurrent herpes simplex labialis. (Used with permission from Berger TG, Dept Dermatology, UCSF. Reproduced from McPhee SJ, Papadakis MA [editors]: Current Medical Diagnosis & Treatment, 48th ed. McGraw-Hill, 2009.)
Recurrent disease is characterized by a cluster of vesicles most commonly localized at the border of the lip (Figure 33-4B). Intense pain occurs at the outset but fades over 4–5 days. Lesions progress through the pustular and crusting stages, and healing without scarring usually completes in 8–10 days. The lesions may recur, repeatedly and at various intervals, in the same location. The frequency of recurrences varies widely among individuals. Many recurrences of oral shedding are asymptomatic and of short duration (24 hours).
HSV infections may occur in the eye, producing severe keratoconjunctivitis. Recurrent lesions of the eye are common and appear as dendritic keratitis or corneal ulcers or as vesicles on the eyelids. With recurrent keratitis, there may be progressive involvement of the corneal stroma, with permanent opacification and blindness. HSV infections are second only to trauma as a cause of corneal blindness in the United States.
Genital disease is more often caused by HSV-2, although HSV-1 can also cause clinical episodes of genital herpes. Primary genital herpes infections can be severe, with illness lasting about 3 weeks. Genital herpes is characterized by vesiculoulcerative lesions of the penis of the male or of the cervix, vulva, vagina, and perineum of the female. The lesions are very painful and may be associated with fever, malaise, dysuria, and inguinal lymphadenopathy. Complications include extragenital lesions (~20% of cases) and aseptic meningitis (~10% of cases). Viral secretion persists for about 3 weeks.
Because of the antigenic cross-reactivity between HSV-1 and HSV-2, preexisting immunity provides some protection against heterotypic infection. An initial HSV-2 infection in a person already immune to HSV-1 tends to be less severe.
Recurrences of genital herpetic infections are common and tend to be mild. A limited number of vesicles appear and heal in about 10 days. Virus is shed for only a few days. Some recurrences are asymptomatic with anogenital shedding lasting less than 24 hours. Whether a recurrence is symptomatic or asymptomatic, a person shedding virus can transmit the infection to sexual partners.
Intact skin is resistant to HSV, so cutaneous HSV infections are uncommon in healthy persons. Localized lesions caused by HSV-1 or HSV-2 may occur in abrasions that become contaminated with the virus (traumatic herpes). These lesions are seen on the fingers of dentists and hospital personnel (herpetic whitlow) and on the bodies of wrestlers (herpes gladiatorum).
Cutaneous infections are often severe and life threatening when they occur in individuals with disorders of the skin, such as eczema or burns, that permit extensive local viral replication and spread. Eczema herpeticum is a primary infection, usually with HSV-1, in a person with chronic eczema. In rare instances, the illness may be fatal.
A severe form of meningitis or encephalitis may be produced by herpesvirus. HSV-1 infections are considered the most common cause of sporadic, fatal encephalitis in the United States. The disease carries a high mortality rate, and those who survive often have residual neurologic defects. About half of patients with HSV encephalitis appear to have primary infections, and the rest appear to have recurrent infection.
HSV infection of the newborn may be acquired in utero, during birth, or after birth. The mother is the most common source of infection in all cases. Neonatal herpes is estimated to occur in about 1 in 5000 deliveries per year. The newborn infant seems to be unable to limit the replication and spread of HSV and has a propensity to develop severe disease.
The most common route of infection (~75% of cases) is for HSV to be transmitted to a newborn during birth by contact with herpetic lesions in the birth canal. To avoid infection, delivery by cesarean section has been used in pregnant women with genital herpes lesions. However, many fewer cases of neonatal HSV infection occur than cases of recurrent genital herpes, even when the virus is present at term.
Neonatal herpes can be acquired postnatally by exposure to either HSV-1 or HSV-2. Sources of infection include family members and hospital personnel who are shedding virus. About 75% of neonatal herpes infections are caused by HSV-2. There do not appear to be any differences between the nature and severity of neonatal herpes in premature or full-term infants, in infections caused by HSV-1 or HSV-2, or in disease when virus is acquired during delivery or postpartum.
Neonatal herpes infections are almost always symptomatic. The overall mortality rate of untreated disease is 50%. Babies with neonatal herpes exhibit three categories of disease: (1) lesions localized to the skin, eye, and mouth; (2) encephalitis with or without localized skin involvement; and (3) disseminated disease involving multiple organs, including the central nervous system. The worst prognosis (~80% mortality rate) applies to infants with disseminated infection, many of whom develop encephalitis. The cause of death of babies with disseminated disease is usually viral pneumonitis or intravascular coagulopathy. Many survivors of severe infections are left with permanent neurologic impairment.
Immunocompromised patients are at increased risk of developing severe HSV infections. These include patients immunosuppressed by disease or therapy (especially those with deficient cellular immunity) and individuals with malnutrition. Renal, cardiac, and bone marrow transplant recipients are at particular risk for severe herpes infections. Patients with hematologic malignancies and patients with AIDS have more frequent and more severe HSV infections. Herpes lesions may spread and involve the respiratory tract, esophagus, and intestinal mucosa. Malnourished children are prone to fatal disseminated HSV infections. In most cases, the disease reflects reactivation of latent HSV infection.
Many newborns acquire passively transferred maternal antibodies. These antibodies are lost during the first 6 months of life, and the period of greatest susceptibility to primary herpes infection occurs between ages 6 months and 2 years. Transplacentally acquired antibodies from the mother are not totally protective against infection of newborns, but they seem to ameliorate infection if not prevent it. HSV-1 antibodies begin to appear in the population in early childhood; by adolescence, they are present in most persons. Antibodies to HSV-2 rise during the age of adolescence and sexual activity.
During primary infections, IgM antibodies appear transiently and are followed by IgG and IgA antibodies that persist for long periods. The more severe the primary infection or the more frequent the recurrences, the greater the level of antibody response. However, the pattern of antibody response has not correlated with the frequency of disease recurrence. Cell-mediated immunity and nonspecific host factors (natural killer cells, interferon) are important in controlling both primary and recurrent HSV infections.
After recovery from a primary infection (inapparent, mild, or severe), the virus is carried in a latent state in the presence of antibodies. These antibodies do not prevent reinfection or reactivation of latent virus but may modify subsequent disease.
Polymerase chain reaction (PCR) assays can be used to detect virus in vesicle swabs, blood, CSF, and tissue and are sensitive and specific. PCR amplification of viral DNA from cerebrospinal fluid is the most sensitive means of detection and is recommended for diagnosis of herpes meningitis/encephalitis.
Virus culture is commonly used, particularly for diagnosis of mucocutaneous disease. Virus may be isolated from herpetic lesions and may also be found in respiratory samples, tissues and body fluids, both during primary infection and during asymptomatic periods. Therefore, the isolation of HSV is not in itself sufficient evidence to indicate that the virus is the causative agent of a disease under investigation.
Inoculation of tissue cultures is used for viral isolation. HSV is easy to cultivate, and cytopathic effects usually occur in only 2–3 days. The agent is then identified by neutralization test or immunofluorescence staining with specific antiserum. Shell vial culture can be used to detect HSV replicating within cells after 24 hours of incubation using fluorescent antibodies. Typing of HSV isolates may be done using monoclonal antibody, sequence analysis, or by restriction endonuclease analysis of viral DNA but is only useful for epidemiologic studies.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree