PHP-IA AND PHP-IB Individuals with PHP-I, the most common of the disorders, show a deficient urinary cAMP response to administration of exogenous PTH. Patients with PHP-I are divided into type Ia and type Ib. Patients with PHP-Ia show evidence for AHO and reduced amounts of Gsα protein/activity, as determined in readily accessible tissues such as erythrocytes, lymphocytes, and fibroblasts. Patients with PHP-Ib typically lack evidence for AHO and they have normal Gsα activity. PHP-Ic, sometimes listed as a third form of PHP-I, is really a variant of PHP-Ia, although the mutant Gsα shows normal activity in certain in vitro assays.
Most patients who have PHP-Ia reveal characteristic features of AHO, which consist of short stature, round face, obesity, skeletal anomalies (brachydactyly), intellectual impairment, and/or heterotopic calcifications. Patients have low calcium and high phosphate levels, as with true hypoparathyroidism. PTH levels, however, are elevated, reflecting resistance to hormone action.
Amorphous deposits of calcium and phosphate are found in the basal ganglia in about one-half of patients. The defects in metacarpal and metatarsal bones are sometimes accompanied by short phalanges as well, possibly reflecting premature closing of the epiphyses. The typical findings are short fourth and fifth metacarpals and metatarsals. The defects are usually bilateral. Exostoses and radius curvus are frequent.
Inheritance and Genetic Defects Multiple defects at the GNAS locus have now been identified in PHP-Ia, PHP-Ib, and PPHP patients. This gene, which is located on chromosome 20q13.3, encodes the α-subunit of the stimulatory G protein (Gsα), among other products (see below). Mutations include abnormalities in splice junctions associated with deficient mRNA production, point mutations, insertions, and/or deletion that all result in a protein with defective function resulting in a 50% reduction of Gsα activity in erythrocytes or other cells.
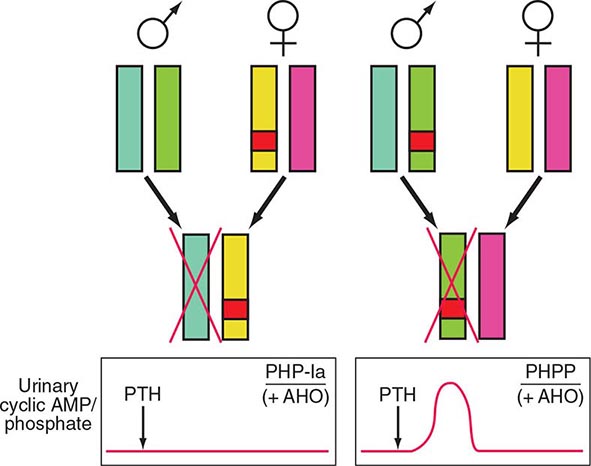
Detailed analyses of disease transmission in affected kindreds have clarified many features of PHP-Ia, PPHP, and PHP-Ib (Fig. 424-7). The former two entities, often traced through multiple generations, have an inheritance pattern consistent with genetic imprinting. The phenomenon of gene imprinting, involving methylation of genetic loci, independent of any mutation, impairs transcription from either the maternal or the paternal allele (Chap. 82). The Gsα transcript is biallelically expressed in most tissues; expression from paternal allele is silenced through as-of-yet unknown mechanisms in some tissues including the proximal renal tubules and the thyroid; consequently, inheritance of a defective paternal allele has no implications with regard to hormonal function. Thus, females affected by either PHP-Ia or PPHP will have offspring with PHP-Ia, if these children inherit the allele carrying the GNAS mutation; in contrast, if the mutant allele is inherited from a male affected by either disorder, the offspring will exhibit PPHP. Consistent with these data in humans, gene-ablation studies in mice have shown that inheritance of the mutant Gsα allele from the female causes much reduced Gsα protein in renal cortex, hypocalcemia, and resistance to PTH. Offspring inheriting the mutant allele from the male showed no evidence of PTH resistance or hypocalcemia.
FIGURE 424-7 Paternal imprinting of renal parathyroid hormone (PTH) resistance. An impaired excretion of urinary cyclic AMP and phosphate is observed in patients with pseudohypoparathyroidism type Ia (PHP-Ia). In the renal cortex, there is selective silencing of paternal Gsα expression. The disease becomes manifest only in patients who inherit the defective gene from an obligate female carrier (left). If the genetic defect is inherited from an obligate male gene carrier, there is no biochemical abnormality; administration of PTH causes an appropriate increase in the urinary cyclic AMP and phosphate concentration (pseudo-PHP [PPHP]; right). Both patterns of inheritance lead to Albright’s hereditary osteodystrophy (AHO), perhaps because of haplotype insufficiency—i.e., both copies of Gsα must be active for normal bone development.
Imprinting is tissue selective. Paternal Gsα expression is not silenced in most tissues. It seems likely, therefore, that the AHO phenotype recognized in PPHP as well as PHP-Ia reflects Gsα haploinsufficiency during embryonic or postnatal development.
The complex mechanisms that control the GNAS gene contribute to challenges involved in unraveling the pathogenesis of these disorders, especially that of PHP-Ib. Much intensive work with families in which multiple members are affected by PHP-Ib, as well as studies of the complex regulation of the GNAS gene locus, have now shown that PHP-Ib is caused by microdeletions within or upstream of the GNAS locus, which are associated with a loss of DNA methylation at one or several loci of the maternal allele (Table 424-6). These abnormalities in methylation silence the expression of the gene. This leads in the proximal renal tubules—where Gsα appears to be expressed exclusively from the maternal allele—to PTH resistance.
PHP-Ib, lacking the AHO phenotype in most instances, shares with PHP-Ia the hypocalcemia and hyperphosphatemia caused by PTH resistance, and thus the blunted urinary cAMP response to administered PTH, a standard test to assess the presence or absence of hormone resistance (Table 424-6). Furthermore, these endocrine abnormalities become apparent only if the disease-causing mutation is inherited maternally. Bone responsiveness may be excessive rather than blunted in PHP-Ib (and in PHP-Ia) patients, based on case reports that have emphasized an osteitis fibrosa–like pattern in several PHP-Ib patients.
PHP-II refers to patients with hypocalcemia and hyperphosphatemia, who have a normal urinary cAMP but an impaired urinary phosphaturic response to PTH. In a PHP-II variant, referred to as acrodysostosis with hormonal resistance (ADOHR), patients have a defect in the regulatory subunit of PKA (PRKAR1A) that mediates the response to PTH distal to cAMP production. Acrodysostosis without hormonal resistance is caused by mutations in the cAMP-selective phosphodiesterase 4 (ADOP4). It remains unclear why the PTH-resistance in some patients, labeled as PHP-II without bony abnormalities, resolves upon treatment with vitamin D supplements.
The diagnosis of these hormone-resistant states can usually be made without difficulty when there is a positive family history for features of AHO, in association with the signs and symptoms of hypocalcemia. In both categories—PHP-Ia and PHP-Ib—serum PTH levels are elevated, particularly when patients are hypocalcemic. However, patients with PHP-Ib or PHP-II without acrodysostosis present only with hypocalcemia and high PTH levels, as evidence for hormone resistance. In PHP-Ia and PHP-Ib, the response of urinary cAMP to the administration of exogenous PTH is blunted. The diagnosis of PHP-II, in the absence of acrodysostosis, is more complex, and vitamin D deficiency must be excluded before such a diagnosis can be entertained.
TREATMENT | PSEUDOHYPOPARATHYROIDISM |
Treatment of PHP is similar to that of hypoparathyroidism, except that calcium and vitamin D doses are usually higher. Patients with PHP show no PTH-resistance in the distal tubules—hence, urinary calcium clearance is typically reduced, and they are not at risk of developing nephrocalcinosis as are patients with true hypoparathyroidism, unless overtreatment occurs, for example, after the completion of pubertal development and skeletal mutation, when calcium and 1,25(OH)2D treatment should be reduced. Variability in response makes it necessary to establish the optimal regimen for each patient, based on maintaining appropriate blood calcium level and urinary calcium excretion and keeping the PTH level within or slightly above the normal range.
PTH OVERWHELMED
Occasionally, loss of calcium from the ECF is so severe that PTH cannot compensate. Such situations include acute pancreatitis and severe, acute hyperphosphatemia, often in association with renal failure, conditions in which there is rapid efflux of calcium from the ECF. Severe hypocalcemia can occur quickly; PTH rises in response to hypocalcemia but does not return blood calcium to normal.
Severe, Acute Hyperphosphatemia Severe hyperphosphatemia is associated with extensive tissue damage or cell destruction (Chap. 423). The combination of increased release of phosphate from muscle and impaired ability to excrete phosphorus because of renal failure causes moderate to severe hyperphosphatemia, the latter causing calcium loss from the blood and mild to moderate hypocalcemia. Hypocalcemia is usually reversed with tissue repair and restoration of renal function as phosphorus and creatinine values return to normal. There may even be a mild hypercalcemic period in the oliguric phase of renal function recovery. This sequence, severe hypocalcemia followed by mild hypercalcemia, reflects widespread deposition of calcium in muscle and subsequent redistribution of some of the calcium to the ECF after phosphate levels return to normal.
Other causes of hyperphosphatemia include hypothermia, massive hepatic failure, and hematologic malignancies, either because of high cell turnover of malignancy or because of cell destruction by chemotherapy.
TREATMENT | SEVERE, ACUTE HYPERPHOSPHATEMIA |
Treatment is directed toward lowering of blood phosphate by the administration of phosphate-binding antacids or dialysis, often needed for the management of CKD. Although calcium replacement may be necessary if hypocalcemia is severe and symptomatic, calcium administration during the hyperphosphatemic period tends to increase extraosseous calcium deposition and aggravate tissue damage. The levels of 1,25(OH)2D may be low during the hyperphosphatemic phase and return to normal during the oliguric phase of recovery.
Osteitis Fibrosa after Parathyroidectomy Severe hypocalcemia after parathyroid surgery is rare now that osteitis fibrosa cystica is an infrequent manifestation of hyperparathyroidism. When osteitis fibrosa cystica is severe, however, bone mineral deficits can be large. After parathyroidectomy, hypocalcemia can persist for days if calcium replacement is inadequate. Treatment may require parenteral administration of calcium; addition of calcitriol and oral calcium supplementation is sometimes needed for weeks to a month or two until bone defects are filled (which, of course, is of therapeutic benefit in the skeleton), making it possible to discontinue parenteral calcium and/or reduce the amount.
DIFFERENTIAL DIAGNOSIS OF HYPOCALCEMIA
Care must be taken to ensure that true hypocalcemia is present; in addition, acute transient hypocalcemia can be a manifestation of a variety of severe, acute illnesses, as discussed above. Chronic hypocalcemia, however, can usually be ascribed to a few disorders associated with absent or ineffective PTH. Important clinical criteria include the duration of the illness, signs or symptoms of associated disorders, and the presence of features that suggest a hereditary abnormality. A nutritional history can be helpful in recognizing a low intake of vitamin D and calcium in the elderly, and a history of excessive alcohol intake may suggest magnesium deficiency.
Hypoparathyroidism and PHP are typically lifelong illnesses, usually (but not always) appearing by adolescence; hence, a recent onset of hypocalcemia in an adult is more likely due to nutritional deficiencies, renal failure, or intestinal disorders that result in deficient or ineffective vitamin D. Neck surgery, even long past, however, can be associated with a delayed onset of postoperative hypoparathyroidism. A history of seizure disorder raises the issue of anticonvulsive medication. Developmental defects may point to the diagnosis of PHP. Rickets and a variety of neuromuscular syndromes and deformities may indicate ineffective vitamin D action, either due to defects in vitamin D metabolism or to vitamin D deficiency.
A pattern of low calcium with high phosphorus in the absence of renal failure or massive tissue destruction almost invariably means hypoparathyroidism or PHP. A low calcium and low phosphorus pattern points to absent or ineffective vitamin D, thereby impairing the action of PTH on calcium metabolism (but not phosphate clearance). The relative ineffectiveness of PTH in calcium homeostasis in vitamin D deficiency, anticonvulsant therapy, gastrointestinal disorders, and hereditary defects in vitamin D metabolism leads to secondary hyperparathyroidism as a compensation. The excess PTH on renal tubule phosphate transport accounts for renal phosphate wasting and hypophosphatemia.
Exceptions to these patterns may occur. Most forms of hypomagnesemia are due to long-standing nutritional deficiency as seen in chronic alcoholics. Despite the fact that the hypocalcemia is principally due to an acute absence of PTH, phosphate levels are usually low, rather than elevated, as in hypoparathyroidism. Chronic renal failure is often associated with hypocalcemia and hyperphosphatemia, despite secondary hyperparathyroidism.
Diagnosis is usually established by application of the PTH immunoassay, tests for vitamin D metabolites, and measurements of the urinary cAMP response to exogenous PTH. In hereditary and acquired hypoparathyroidism and in severe hypomagnesemia, PTH is either undetectable or inappropriately in the normal range (Fig. 424-4). This finding in a hypocalcemic patient is supportive of hypoparathyroidism, as distinct from ineffective PTH action, in which even mild hypocalcemia is associated with elevated PTH levels. Hence a failure to detect elevated PTH levels establishes the diagnosis of hypoparathyroidism; elevated levels suggest the presence of secondary hyperparathyroidism, as found in many of the situations in which the hormone is ineffective due to associated abnormalities in vitamin D action. Assays for 25(OH)D can be helpful. Low or low-normal 25(OH)D indicates vitamin D deficiency due to lack of sunlight, inadequate vitamin D intake, or intestinal malabsorption. Recognition that mild hypocalcemia, rickets, and hypophosphatemia are due to anticonvulsant therapy is made by history.
TREATMENT | HYPOCALCEMIC STATES |
The management of hypoparathyroidism, PHP, chronic renal failure, and hereditary defects in vitamin D metabolism involves the use of vitamin D or vitamin D metabolites and calcium supplementation. Vitamin D itself is the least expensive form of vitamin D replacement and is frequently used in the management of uncomplicated hypoparathyroidism and some disorders associated with ineffective vitamin D action. When vitamin D is used prophylactically, as in the elderly or in those with chronic anticonvulsant therapy, there is a wider margin of safety than with the more potent metabolites. However, most of the conditions in which vitamin D is administered chronically for hypocalcemia require amounts 50–100 times the daily replacement dose because the formation of 1,25(OH)2D is deficient. In such situations, vitamin D is no safer than the active metabolite because intoxication can occur with high-dose therapy (because of storage in fat). Calcitriol is more rapid in onset of action and also has a short biologic half-life.
Vitamin D (at least 1000 U/d [2–3 μg/d] [higher levels required in older persons]) or calcitriol (0.25–1 μg/d) is required to prevent rickets in normal individuals. In contrast, 40,000–120,000 U (1–3 mg) of vitamin D2 or D3 is typically required in hypoparathyroidism. The dose of calcitriol is unchanged in hypoparathyroidism, because the defect is in hydroxylation by the 25(OH)D-1α-hydroxylase. Calcitriol is also used in disorders of 25(OH)D-1α-hydroxylase; vitamin D receptor defects are much more difficult to treat.
Patients with hypoparathyroidism should be given 2–3 g of elemental calcium PO each day. The two agents, vitamin D or calcitriol and oral calcium, can be varied independently. Urinary calcium excretion needs to be monitored carefully. If hypocalcemia alternates with episodes of hypercalcemia in high-brittleness patients with hypoparathyroidism, administration of calcitriol and use of thiazides, as discussed above, may make management easier. Clinical trials with PTH(1–34) or PTH(1–84) are promising, but these alternative treatments have not yet been approved.
425 | Osteoporosis |
Osteoporosis, a condition characterized by decreased bone strength, is prevalent among postmenopausal women but also occurs in men and women with underlying conditions or major risk factors associated with bone demineralization. Its chief clinical manifestations are vertebral and hip fractures, although fractures can occur at almost any skeletal site. Osteoporosis affects almost 10 million individuals in the United States, but only a small proportion are diagnosed and treated.
DEFINITION
Osteoporosis is defined as a reduction in the strength of bone that leads to an increased risk of fractures. Loss of bone tissue is associated with deterioration in skeletal microarchitecture. The World Health Organization (WHO) operationally defines osteoporosis as a bone density that falls 2.5 standard deviations (SD) below the mean for young healthy adults of the same sex—also referred to as a T-score of –2.5. Postmenopausal women who fall at the lower end of the young normal range (a T-score <–1.0) are defined as having low bone density and are also at increased risk of osteoporosis. Although risk is lower in this group, more than 50% of fractures among postmenopausal women, including hip fractures, occur in this group with low bone density, because the number of individuals in this category is so much larger than that in the osteoporosis range. As a result, there are ongoing attempts to identify individuals within the low bone density range who are at high risk of fracture and might benefit from pharmacologic intervention. Furthermore, some have advocated using fracture risk as the “diagnostic” criterion for osteoporosis.
EPIDEMIOLOGY
In the United States, as many as 9 million adults have osteoporosis (T-score <–2.5 in either spine or hip), and an additional 48 million individuals have bone mass levels that put them at increased risk of developing osteoporosis (e.g., bone mass T-score <–1.0). Osteoporosis occurs more frequently with increasing age as bone tissue is lost progressively. In women, the loss of ovarian function at menopause (typically about age 50) precipitates rapid bone loss so that most women meet the diagnostic criterion for osteoporosis by age 70–80. As the population continues to age, the number of individuals with osteoporosis and fractures will also continue to increase, despite a recognized reduction in age-specific risk. It is estimated that about 2 million fractures occur each year in the United States as a consequence of osteoporosis, and that number is expected to increase as the population continues to age.
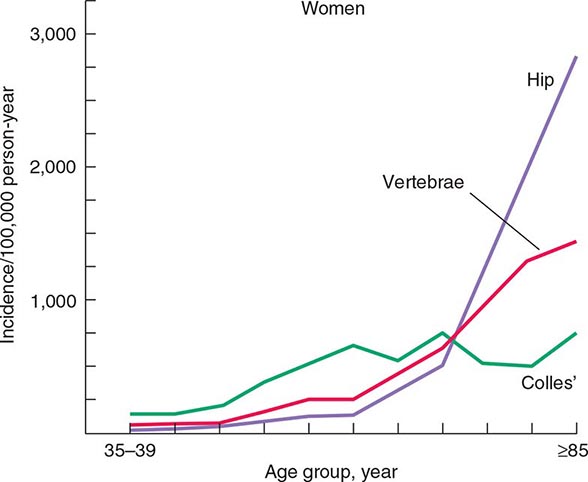
The epidemiology of fractures follows the trend for loss of bone density, with exponential increases in both hip and vertebral fractures with age. Fractures of the distal radius have a somewhat different epidemiology, increasing in frequency before age 50 and plateauing by age 60, with only a modest age-related increase thereafter. In contrast, incidence rates for hip fractures double every 5 years after age 70 (Fig. 425-1). This distinct epidemiology may be related to the way the elderly fall as they age, with fewer falls on an outstretched hand and more falls directly on the hip. About 300,000 hip fractures occur each year in the United States, most of which require hospital admission and surgical intervention. The probability that a 50-year-old white individual will have a hip fracture during his or her lifetime is 14% for women and 5% for men; the risk for African Americans is lower (about one-half those rates), and the risk for Asians is roughly equal to that for whites. Hip fractures are associated with a high incidence of deep vein thrombosis and pulmonary embolism (20–50%) and a mortality rate between 5 and 20% during the year after surgery. there is also significant morbidity, with about 20–40% of survivors requiring long-term care, and many who are unable to function as they did before the fracture.
FIGURE 425-1 Epidemiology of vertebral, hip, and Colles’ fractures with age. (Adapted from C Cooper, LJ Melton III: Trends Endocrinol Metab 3:224, 1992; with permission.)
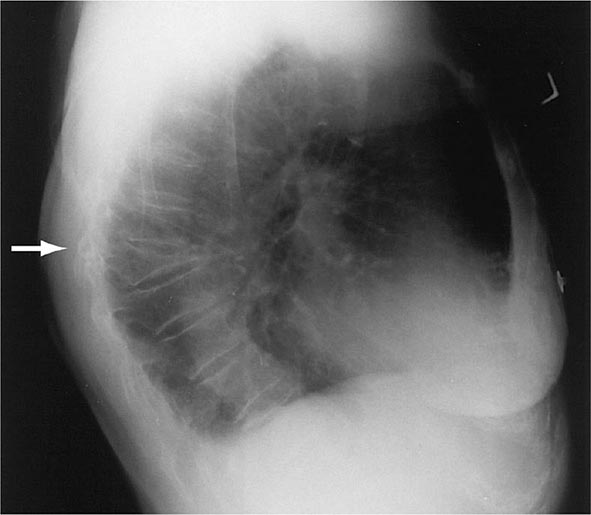
There are about 550,000 vertebral crush fractures per year in the United States. Only a fraction (estimated to be one-third) of them are recognized clinically, because many are relatively asymptomatic and are identified incidentally during radiography for other purposes (Fig. 425-2). Vertebral fractures rarely require hospitalization but are associated with long-term morbidity and a slight increase in mortality rates, primarily related to pulmonary disease. Multiple vertebral fractures lead to height loss (often of several inches), kyphosis, and secondary pain and discomfort related to altered biomechanics of the back. Thoracic fractures can be associated with restrictive lung disease, whereas lumbar fractures are associated with abdominal symptoms that include distention, early satiety, and constipation.
FIGURE 425-2 Lateral spine x-ray showing severe osteopenia and a severe wedge-type deformity (severe anterior compression).
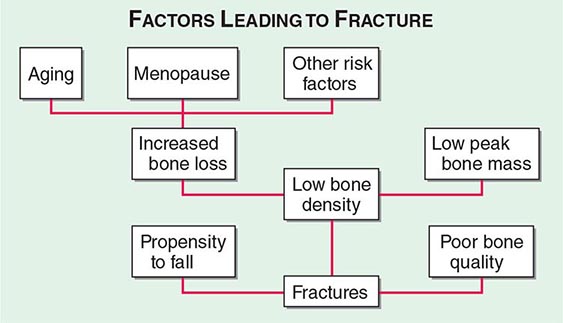
Approximately 400,000 wrist fractures and 135,000 pelvic fractures occur in the United States each year. Fractures of the humerus and other bones (estimated to be about 675,000 per year) also occur with osteoporosis; this is not surprising in light of the fact that bone loss is a systemic phenomenon. Although some fractures result from major trauma, the threshold for fracture is reduced for an osteoporotic bone (Fig. 425-3). In addition to bone density, there are a number of risk factors for fracture; the common ones are summarized in Table 425-1 Age, prior fractures (especially recent fractures), a family history of osteoporosis-related fractures, low body weight, smoking, and excessive alcohol use are all independent predictors of fracture. Chronic diseases with inflammatory components that increase skeletal remodeling such as rheumatoid arthritis, increase the risk of osteoporosis, as do diseases associated with malabsorption. Chronic diseases that increase the risk of falling or frailty, including dementia, Parkinson’s disease, and multiple sclerosis, also increase fracture risk.
FIGURE 425-3 Factors leading to osteoporotic fractures.
CONDITIONS, DISEASES, AND MEDICATIONS THAT CONTRIBUTE TO OSTEOPOROSIS AND FRACTURES |
In the United States and Europe, osteoporosis-related fractures are more common among women than men, presumably due to a lower peak bone mass as well as postmenopausal bone loss in women. However, this sex difference in bone density and age-related increase in hip fractures is not as apparent in some other cultures, possibly due to genetics, physical activity level, or diet.
Fractures are themselves risk factors for future fractures (Table 425-1). Vertebral fractures increase the risk of other vertebral fractures as well as fractures of the peripheral skeleton such as the hip and wrist. Wrist fractures also increase the risk of vertebral and hip fractures. The risk for subsequent fractures is particularly high in the first several years after the first fracture, and the risk wanes considerably thereafter. Consequently, among individuals over age 50, any fracture should be considered as potentially related to osteoporosis regardless of the circumstances of the fracture. Osteoporotic bone is more likely to fracture than normal bone at any level of trauma, and a fracture in a person over 50 should trigger evaluation for osteoporosis. This often does not occur because postfracture care is not always well coordinated.
PATHOPHYSIOLOGY
BONE REMODELING
Osteoporosis results from bone loss due to age-related changes in bone remodeling as well as extrinsic and intrinsic factors that exaggerate this process. These changes may be superimposed on a low peak bone mass. Consequently, understanding the bone remodeling process is fundamental to understanding the pathophysiology of osteoporosis (Chap. 423). During growth, the skeleton increases in size by linear growth and by apposition of new bone tissue on the outer surfaces of the cortex (Fig. 425-4). The latter process is called modeling, a process that also allows the long bones to adapt in shape to the stresses placed on them. Increased sex hormone production at puberty is required for skeletal maturation, which reaches maximum mass and density in early adulthood. It is around puberty that the sexual dimorphism in skeletal size becomes obvious, although true bone density remains similar between the sexes. Nutrition and lifestyle also play an important role in growth, although genetic factors primarily determine peak skeletal mass and density. Numerous genes control skeletal growth, peak bone mass, and body size, as well as skeletal structure and density. Heritability estimates of 50–80% for bone density and size have been derived on the basis of twin studies. Although peak bone mass is often lower among individuals with a family history of osteoporosis, association studies of candidate genes (vitamin D receptor; type I collagen, the estrogen receptor [ER], and interleukin 6 [IL-6]; and insulin-like growth factor I [IGF-I]) and bone mass, bone turnover, and fracture prevalence have been inconsistent. Linkage studies suggest that a genetic locus on chromosome 11 is associated with high bone mass. Families with high bone mass and without much apparent age-related bone loss have been shown to have a point mutation in LRP5, a low-density lipoprotein receptor–related protein. The role of this gene in the general population is not clear, although a nonfunctional mutation results in osteoporosis-pseudoglioma syndrome, and LRP5 signaling appears to be important in controlling bone formation. LRP5 acts through the Wnt signaling pathway. With LRP5 and Wnt activation, beta-catenin is translocated to the nucleus, allowing stimulation of osteoblast formation, activation, and life span as well as suppression of osteoclast activity, thereby increasing bone formation. The osteocyte product, sclerostin, is a negative inhibitor of Wnt signaling.
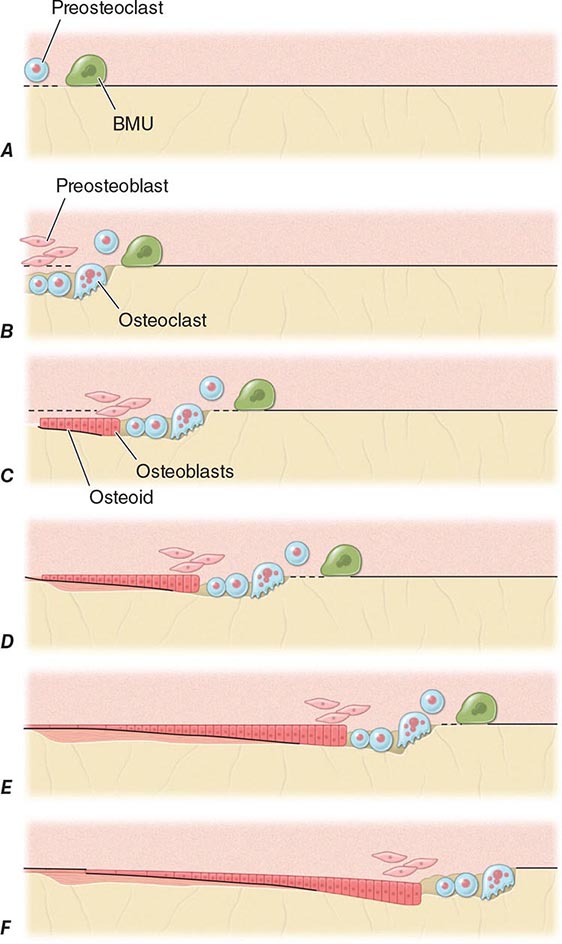
FIGURE 425-4 Mechanism of bone remodeling. The basic molecular unit (BMU) moves along the trabecular surface at a rate of about 10 μm/d. The figure depicts remodeling over ~120 days. A. Origination of BMU-lining cells contracts to expose collagen and attract preosteoclasts. B. Osteoclasts fuse into multinucleated cells that resorb a cavity. Mononuclear cells continue resorption, and preosteoblasts are stimulated to proliferate. C. Osteoblasts align at bottom of cavity and start forming osteoid (black). D. Osteoblasts continue formation and mineralization. Previous osteoid starts to mineralize (horizontal lines). E. Osteoblasts begin to flatten. F. Osteoblasts turn into lining cells; bone remodeling at initial surface (left of drawing) is now complete, but BMU is still advancing (to the right). (Adapted from SM Ott, in JP Bilezikian et al [eds]: Principles of Bone Biology, vol. 18. San Diego, Academic Press, 1996, pp 231–241.)
Genome-wide scans for low bone mass suggest multiple genes are involved, many of which are also implicated in control of body size.
In adults, bone remodeling, not modeling, is the principal metabolic skeletal process. Bone remodeling has two primary functions: (1) to repair microdamage within the skeleton to maintain skeletal strength and ensure the relative youth of the skeleton and (2) to supply calcium from the skeleton to maintain serum calcium. Remodeling may be activated by microdamage to bone as a result of excessive or accumulated stress. Acute demands for calcium involve osteoclast-mediated resorption as well as calcium transport by osteocytes. Chronic demands for calcium result in secondary hyperparathyroidism, increased bone remodeling, and overall loss of bone tissue.
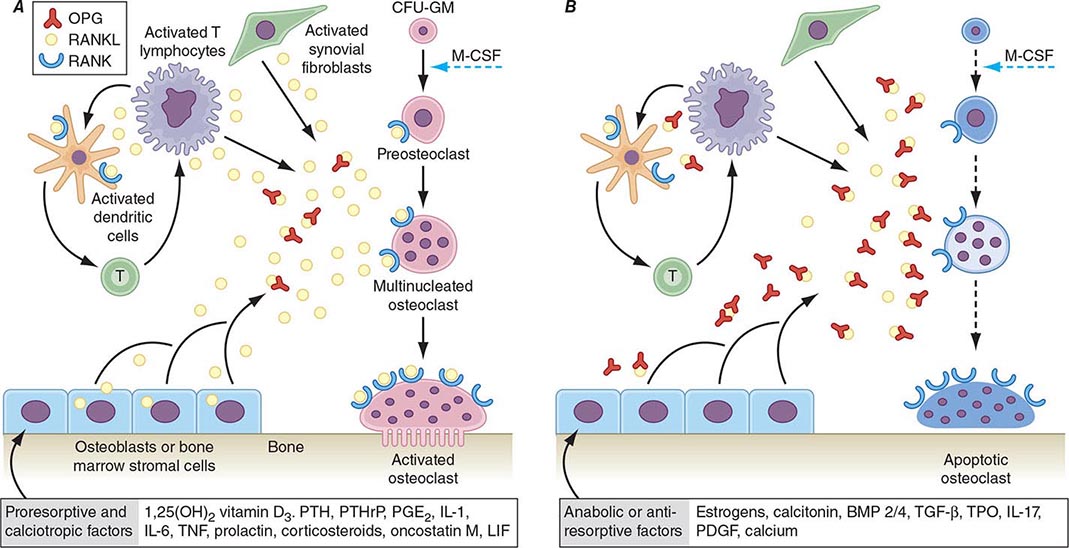
Bone remodeling also is regulated by several circulating hormones, including estrogens, androgens, vitamin D, and parathyroid hormone (PTH), as well as locally produced growth factors such as IGF-I and immunoreactive growth hormone II (IGH-II), transforming growth factor β (TGF-β), parathyroid hormone–related peptide (PTHrP), interleukins (ILs), prostaglandins, and members of the tumor necrosis factor (TNF) superfamily. These factors primarily modulate the rate at which new remodeling sites are activated, a process that results initially in bone resorption by osteoclasts, followed by a period of repair during which new bone tissue is synthesized by osteoblasts. The cytokine responsible for communication between the osteoblasts, other marrow cells, and osteoclasts is RANK ligand (RANKL; receptor activator of nuclear factor-κB [NF-κB]). RANKL, a member of the TNF family, is secreted by osteoblasts and certain cells of the immune system (Chap. 423). The osteoclast receptor for this protein is referred to as RANK. Activation of RANK by RANKL is a final common path in osteoclast development, activation, and life span. A humoral decoy for RANKL, also secreted by osteoblasts, is osteoprotegerin (Fig. 425-5). Modulation of osteoclast recruitment and activity appears to be related to the interplay among these three factors. It appears that estrogens are pivotal in modulating secretion of osteoprotegerin (OPG) and perhaps also RANKL. Additional influences include nutrition (particularly calcium intake) and physical activity level.
FIGURE 425-5 Hormonal control of bone resorption. A. Proresorptive and calciotropic factors. B. Anabolic and antiosteoclastic factors. RANK ligand (RANKL) expression is induced in osteoblasts, activated T cells, synovial fibroblasts, and bone marrow stromal cells. It binds to membrane-bound receptor RANK to promote osteoclast differentiation, activation, and survival. Conversely, osteoprotegerin (OPG) expression is induced by factors that block bone catabolism and promote anabolic effects. OPG binds and neutralizes RANKL, leading to a block in osteoclastogenesis and decreased survival of preexisting osteoclasts. CFU-GM, colony-forming units, granulocyte macrophage; IL, interleukin; LIF, leukemia inhibitory factor; M-CSF, macrophage colony-stimulating factor; OPG-L, osteoprotegerin-ligand; PDGF, platelet-derived growth factor; PGE2, prostaglandin E2; PTH, parathyroid hormone; RANKL, receptor activator of nuclear factor nuclear factor-κB; TGF-β, transforming growth factor β; TNF, tumor necrosis factor; TPO, thrombospondin. (From WJ Boyle et al: Nature 423: 337, 2003.)
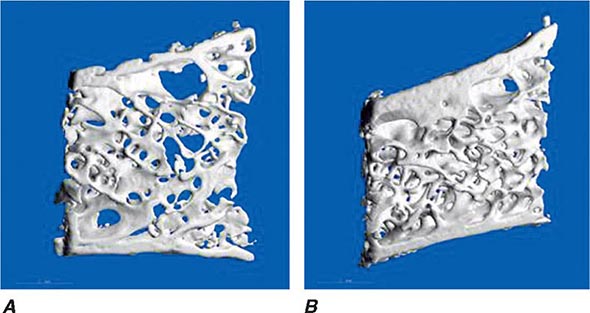
In young adults, resorbed bone is replaced by an equal amount of new bone tissue. Thus, the mass of the skeleton remains constant after peak bone mass is achieved in adulthood. After age 30–45, however, the resorption and formation processes become imbalanced, and resorption exceeds formation. This imbalance may begin at different ages and varies at different skeletal sites; it becomes exaggerated in women after menopause. Excessive bone loss can be due to an increase in osteoclastic activity and/or a decrease in osteoblastic activity. In addition, an increase in remodeling activation frequency, and thus the number of remodeling sites, can magnify the small imbalance seen at each remodeling unit. Increased recruitment of bone remodeling sites produces a reversible reduction in bone tissue but also can result in permanent loss of tissue and disrupted skeletal architecture. In trabecular bone, if the osteoclasts penetrate trabeculae, they leave no template for new bone formation to occur, and, consequently, rapid bone loss ensues and cancellous connectivity becomes impaired. A higher number of remodeling sites increases the likelihood of this event. In cortical bone, increased activation of remodeling creates more porous bone. The effect of this increased porosity on cortical bone strength may be modest if the overall diameter of the bone is not changed. However, decreased apposition of new bone on the periosteal surface coupled with increased endocortical resorption of bone decreases the biomechanical strength of long bones. Even a slight exaggeration in normal bone loss increases the risk of osteoporosis-related fractures because of the architectural changes that occur, and osteoporosis is primarily a disease of disordered skeletal architecture. The main clinically available tool (dual-energy x-ray absorptiometry) measures mass not architecture. Emerging data from high-resolution peripheral quantitative computed tomography (CT) scans suggest that aging is associated with changes in microstructure of bone tissue, including increased cortical porosity and reduced cortical thickness.
CALCIUM NUTRITION
Peak bone mass may be impaired by inadequate calcium intake during growth among other nutritional factors (calories, protein, and other minerals), leading to increased risk of osteoporosis later in life. During the adult phase of life, insufficient calcium intake contributes to relative secondary hyperparathyroidism and an increase in the rate of bone remodeling to maintain normal serum calcium levels. PTH stimulates the hydroxylation of vitamin D in the kidney, leading to increased levels of 1,25-dihydroxyvitamin D [1,25(OH)2D] and enhanced gastrointestinal calcium absorption. PTH also reduces renal calcium loss. Although these are all appropriate compensatory homeostatic responses for adjusting calcium economy, the long-term effects are detrimental to the skeleton because the increased remodeling rates and the ongoing imbalance between resorption and formation at remodeling sites combine to accelerate loss of bone tissue.
Total daily calcium intakes <400 mg are detrimental to the skeleton, and intakes in the range of 600–800 mg, which is about the average intake among adults in the United States, are also probably suboptimal. The recommended daily required intake of 1000–1200 mg for adults accommodates population heterogeneity in controlling calcium balance (Chap. 95e). Such intakes should preferentially come from dietary sources, and supplements should be used only when dietary intakes fall short. The supplement should contain enough calcium to bring total intake to about 1200 mg/d.
VITAMIN D
(See also Chap. 423) Severe vitamin D deficiency causes rickets in children and osteomalacia in adults. However, there is accumulating evidence that vitamin D insufficiency may be more prevalent than previously thought, particularly among individuals at increased risk such as the elderly; those living in northern latitudes; and individuals with poor nutrition, malabsorption, or chronic liver or renal disease. Dark-skinned individuals are also at high risk of vitamin D deficiency. There is controversy regarding optimal levels of serum 25-hydroxy-vitamin D [25(OH)D], with some advocating levels >20 ng/mL and others advocating optimal targets >75 nmol/L (30 ng/mL). To achieve this level for most adults requires an intake of 800–1000 units/d, particularly in individuals who avoid sunlight or routinely use ultra-violet-blocking lotions. Vitamin D insufficiency leads to compensatory secondary hyperparathyroidism and is an important risk factor for osteoporosis and fractures. Some studies have shown that >50% of inpatients on a general medical service exhibit biochemical features of vitamin D deficiency, including increased levels of PTH and alkaline phosphatase and lower levels of ionized calcium. In women living in northern latitudes, vitamin D levels decline during the winter months. This is associated with seasonal bone loss, reflecting increased bone turnover. Even among healthy ambulatory individuals, mild vitamin D deficiency is increasing in prevalence, in part due to decreased exposure to sunlight coupled with increased use of potent sunscreens. Treatment with vitamin D can return levels to normal and prevent the associated increase in bone remodeling, bone loss, and fractures. Improved muscle function and gait associated with reduced falls and fracture rates also have been documented among individuals in northern latitudes who have greater vitamin D intake and higher 25(OH)D levels (see below). Vitamin D adequacy also may affect risk and/or severity of other diseases, including cancers (colorectal, prostate, and breast), autoimmune diseases, and diabetes; however, many observational studies suggesting these potential extraskeletal benefits have not been confirmed with randomized controlled trials.
ESTROGEN STATUS
Estrogen deficiency probably causes bone loss by two distinct but interrelated mechanisms: (1) activation of new bone remodeling sites and (2) exaggeration of the imbalance between bone formation and resorption. The change in activation frequency causes a transient bone loss until a new steady state between resorption and formation is achieved. The remodeling imbalance, however, results in a permanent decrement in mass. In addition, the very presence of more remodeling sites in the skeleton increases the probability that trabeculae will be penetrated, eliminating the template on which new bone can be formed and accelerating the loss of bony tissue.
The most common estrogen-deficient state is the cessation of ovarian function at the time of menopause, which occurs on average at age 51 (Chap. 413). Thus, with current life expectancy, an average woman will spend about 30 years without an ovarian supply of estrogen. The mechanism by which estrogen deficiency causes bone loss is summarized in Fig. 425-5. Marrow cells (macrophages, monocytes, osteoclast precursors, mast cells) as well as bone cells (osteoblasts, osteocytes, osteoclasts) express ERs α and β. Loss of estrogen increases production of RANKL and may reduce production of OPG, increasing osteoclast recruitment. Estrogen also may play an important role in determining the life span of bone cells by controlling the rate of apoptosis. Thus, in situations of estrogen deprivation, the life span of osteoblasts may be decreased, whereas the longevity and activity of osteoclasts are increased. The rate and duration of bone loss after menopause are heterogeneous and unpredictable. Once surfaces are lost in cancellous bone, the rate of bone loss must decline. In cortical bone, loss is slower but continues for a longer time period.
Because remodeling is initiated at the surface of bone, it follows that trabecular bone—which has a considerably larger surface area (80% of the total) than cortical bone—will be affected preferentially by estrogen deficiency. Fractures occur earliest at sites where trabecular bone contributes most to bone strength; consequently, vertebral fractures are the most common early consequence of estrogen deficiency.
PHYSICAL ACTIVITY
Inactivity, such as prolonged bed rest or paralysis, results in significant bone loss. Concordantly, athletes have higher bone mass than does the general population. These changes in skeletal mass are most marked when the stimulus begins during growth and before the age of puberty. Adults are less capable than children of increasing bone mass after restoration of physical activity. Epidemiologic data support the beneficial effects on the skeleton of chronic high levels of physical activity. Fracture risk is lower in rural communities and in countries where physical activity is maintained into old age. However, when exercise is initiated during adult life, the effects of moderate exercise on the skeleton are modest, with a bone mass increase of 1–2% in short-term studies of <2 years in duration. It is argued that more active individuals are less likely to fall and are more capable of protecting themselves upon falling, thereby reducing fracture risk.
CHRONIC DISEASE
Various genetic and acquired diseases are associated with an increase in the risk of osteoporosis (Table 425-1). Mechanisms that contribute to bone loss are unique for each disease and typically result from multiple factors, including nutrition, reduced physical activity levels, and factors that affect rates of bone remodeling. In most, but not all, circumstances the primary diagnosis is made before osteoporosis presents clinically.
MEDICATIONS
A large number of medications used in clinical practice have potentially detrimental effects on the skeleton (Table 425-1). Glucocorticoids are the most common cause of medication-induced osteoporosis. It is often not possible to determine the extent to which osteoporosis is related to glucocorticoids or to other factors, because treatment is superimposed on the effects of the primary disease, which in itself may be associated with bone loss (e.g., rheumatoid arthritis). Excessive doses of thyroid hormone can accelerate bone remodeling and result in bone loss.
Other medications have less detrimental effects on the skeleton than pharmacologic doses of glucocorticoids. Anticonvulsants are thought to increase the risk of osteoporosis, although many affected individuals have concomitant insufficiency of 1,25(OH)2D, as some anticonvulsants induce the cytochrome P450 system and vitamin D metabolism. Patients undergoing transplantation are at high risk for rapid bone loss and fracture not only from glucocorticoids but also from treatment with other immunosuppressants such as cyclosporine and tacrolimus (FK506). In addition, these patients often have underlying metabolic abnormalities, such as hepatic or renal failure, that predispose to bone loss.
Aromatase inhibitors, which potently block the aromatase enzyme that converts androgens and other adrenal precursors to estrogen, reduce circulating postmenopausal estrogen levels dramatically. These agents, which are used in various stages for breast cancer treatment, also have been shown to have a detrimental effect on bone density and risk of fracture. More recently a variety of agents have been implicated in increased bone loss and fractures. These include selective serotonin reuptake inhibitors, proton pump inhibitors, and thiazolidinediones. It is difficult in some cases to separate the risk accrued by the underlying disease from that attributable to the medication. For example, both depression and diabetes are risk factors for fracture by themselves.
CIGARETTE CONSUMPTION
The use of cigarettes over a long period has detrimental effects on bone mass. These effects may be mediated directly by toxic effects on osteoblasts or indirectly by modifying estrogen metabolism. On average, cigarette smokers reach menopause 1–2 years earlier than the general population. Cigarette smoking also produces secondary effects that can modulate skeletal status, including intercurrent respiratory and other illnesses, frailty, decreased exercise, poor nutrition, and the need for additional medications (e.g., glucocorticoids for lung disease).
MEASUREMENT OF BONE MASS
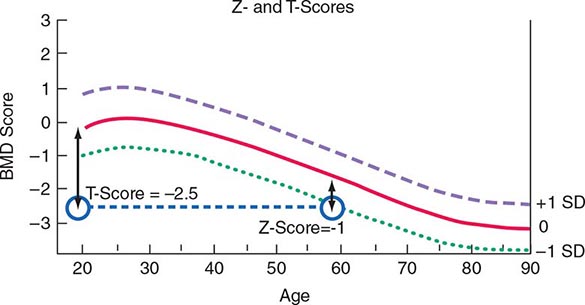
Several noninvasive techniques are available for estimating skeletal mass or density. They include dual-energy x-ray absorptiometry (DXA), single-energy x-ray absorptiometry (SXA), quantitative CT, and ultrasound (US). DXA is a highly accurate x-ray technique that has become the standard for measuring bone density. Although it can be used for measurement in any skeletal site, clinical determinations usually are made of the lumbar spine and hip. DXA also can be used to measure body composition. In the DXA technique, two x-ray energies are used to estimate the area of mineralized tissue, and the mineral content is divided by the area, which partially corrects for body size. However, this correction is only partial because DXA is a two-dimensional scanning technique and cannot estimate the depth or posteroanterior length of the bone. Thus, small slim people tend to have lower than average bone mineral density (BMD), a feature that is important in interpreting BMD measurements when performed in young adults, and something that must be taken into account at any age. Bone spurs, which are common in osteoarthritis, tend to falsely increase bone density of the spine and are a particular problem in measuring the spine in older individuals. Because DXA instrumentation is provided by several different manufacturers, the output varies in absolute terms. Consequently, it has become standard practice to relate the results to “normal” values by using T-scores (a T-score of 1 equals 1 SD), which compare individual results to those in a young population that is matched for race and sex. Z-scores (also measured in SD) compare individual results to those of an age-matched population that also is matched for race and sex. Thus, a 60-year-old woman with a Z-score of –1 (1 SD below mean for age) has a T-score of –2.5 (2.5 SD below mean for a young control group) (Fig. 425-6). A T-score below –2.5 in the lumbar spine, femoral neck, or total hip has been defined as a diagnosis of osteoporosis. As noted above, because more than 50% of fractures occur in individuals with low bone mass rather than BMD osteoporosis, attempts are ongoing to redefine the disease as a fracture risk rather than a specific BMD. Consistent with this concept, fractures of the spine and hip that occur in the absence of major trauma would be considered to be sufficient to diagnose osteoporosis, regardless of BMD. Fractures of other sites, such as pelvis, proximal humerus, and wrist, would be tantamount to an osteoporosis diagnosis in the presence of low BMD. CT can also be used to measure the spine and the hip, but is rarely used clinically, in part because of higher radiation exposure and cost, in addition to a lesser body of data confirming its ability to predict fracture risk, compared with BMD by DXA. High-resolution peripheral CT is used to measure bone in the forearm or tibia as a research tool to noninvasively provide some measure of skeletal architecture. Magnetic resonance imaging (MRI) can also be used in research settings to obtain some architectural information on the forearm and perhaps the hip.
FIGURE 425-6 Relationship between Z-scores and T-scores in a 60-year-old woman. BMD, bone mineral density; SD, standard deviation.
DXA equipment can also be used to obtain lateral images of the spine, from T4 through L4, a technique called vertebral fracture assessment (VFA). Although not as definitive as radiography, it is a useful screening tool when height loss, back pain, or postural change suggests the presence of an undiagnosed vertebral fracture. Furthermore, because vertebral fractures are so prevalent with advancing age, screening vertebral imaging is recommended in women and men with low bone mass (T-score <1) by age 70 and 80, respectively.
US is used to measure bone mass by calculating the attenuation of the signal as it passes through bone or the speed with which it traverses the bone. It is unclear whether US assesses properties of bone other than mass (e.g., quality), but this is a potential advantage of the technique. Because of its relatively low cost and mobility, US is amenable for use as a screening procedure in stores or at health fairs.
All of these techniques for measuring BMD have been approved by the U.S. Food and Drug Administration (FDA) on the basis of their capacity to predict fracture risk. The hip is the preferred site of measurement in most individuals, because it predicts the risk of hip fracture, the most important consequence of osteoporosis, better than any other bone density measurement site. When hip measurements are performed by DXA, the spine can be measured at the same time. In younger individuals such as perimenopausal or early postmenopausal women, spine measurements may be the most sensitive indicator of bone loss. A risk assessment tool (FRAX) incorporates femoral neck BMD to assess 10-year fracture risk (see below).
WHEN TO MEASURE BONE MASS
Clinical guidelines have been developed for the use of bone densitometry in clinical practice. The original National Osteoporosis Foundation guidelines recommend bone mass measurements in postmenopausal women, assuming they have one or more risk factors for osteoporosis in addition to age, sex, and estrogen deficiency. The guidelines further recommend that bone mass measurement be considered in all women by age 65, a position ratified by the U.S. Preventive Health Services Task Force. Criteria approved for Medicare reimbursement of BMD are summarized in Table 425-2.
INDICATIONS FOR BONE DENSITY TESTING |
Source: From the 2014 National Osteoporosis Foundation Clinician’s Guide to the Prevention and Treatment of Osteoporosis. © National Osteoporosis Foundation.
WHEN TO TREAT BASED ON BONE MASS RESULTS
Most guidelines suggest that patients be considered for treatment when BMD is >2.5 SD below the mean value for young adults (T-score ≤–2.5), in either spine, total hip, or femoral neck. Treatment also should also be considered in postmenopausal women with fracture risk factors even if BMD is not in the osteoporosis range. Risk factors (age, prior fracture, family history of hip fracture, low body weight, cigarette consumption, excessive alcohol use, steroid use, and rheumatoid arthritis) can be combined with BMD to assess the likelihood of a fracture over a 5- or 10-year period. Treatment threshold depends on cost-effectiveness analyses but probably is ~1% per year of risk in the United States.
TREATMENT | OSTEOPOROSIS |
MANAGEMENT OF PATIENTS WITH FRACTURES
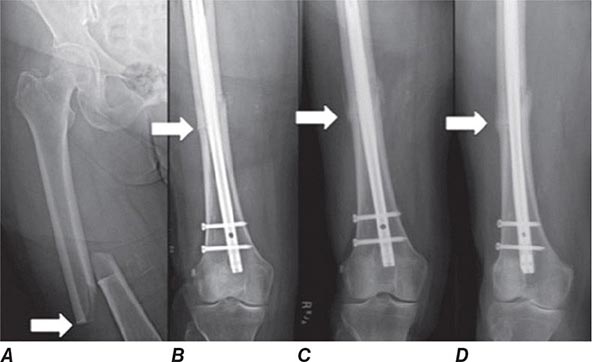
Treatment of a patient with osteoporosis frequently involves management of acute fractures as well as treatment of the underlying disease. Hip fractures almost always require surgical repair if the patient is to become ambulatory again. Depending on the location and severity of the fracture, condition of the neighboring joint, and general status of the patient, procedures may include open reduction and internal fixation with pins and plates, hemiarthroplasties, and total arthroplasties. These surgical procedures are followed by intense rehabilitation in an attempt to return patients to their prefracture functional level. Long bone fractures (e.g., wrist) often require either external or internal fixation. Other fractures (e.g., vertebral, rib, and pelvic fractures) usually are managed with supportive care, requiring no specific orthopedic treatment.
Only ~25–30% of vertebral compression fractures present with sudden-onset back pain. For acutely symptomatic fractures, treatment with analgesics is required, including nonsteroidal anti-inflammatory agents and/or acetaminophen, sometimes with the addition of a narcotic agent (codeine or oxycodone). A few small, randomized clinical trials suggest that calcitonin may reduce pain related to acute vertebral compression fracture. Percutaneous injection of artificial cement (polymethylmethacrylate) into the vertebral body (vertebroplasty or kyphoplasty) may offer significant immediate pain relief in patients with severe pain from acute or subacute vertebral fractures. Safety concerns include extravasation of cement with neurologic sequelae and increased risk of fracture in neighboring vertebrae due to mechanical rigidity of the treated bone. Exactly which patients are the optimal candidates for this procedure remains unknown. Short periods of bed rest may be helpful for pain management, but in general, early mobilization is recommended because it helps prevent further bone loss associated with immobilization. Occasionally, use of a soft elastic-style brace may facilitate earlier mobilization. Muscle spasms often occur with acute compression fractures and can be treated with muscle relaxants and heat treatments.
Severe pain usually resolves within 6–10 weeks. More chronic severe pain might suggest the possibility of multiple myeloma or underlying metastatic disease. Chronic pain following vertebral fracture is probably not bony in origin; instead, it is related to abnormal strain on muscles, ligaments, and tendons and to secondary facet-joint arthritis associated with alterations in thoracic and/or abdominal shape. Chronic pain is difficult to treat effectively and may require analgesics, sometimes including narcotic analgesics. Frequent intermittent rest in a supine or semireclining position is often required to allow the soft tissues, which are under tension, to relax. Back-strengthening exercises (paraspinal) may be beneficial. Heat treatments help relax muscles and reduce the muscular component of discomfort. Various physical modalities, such as US and transcutaneous nerve stimulation, may be beneficial in some patients. Pain also occurs in the neck region, not as a result of compression fractures (which almost never occur in the cervical spine as a result of osteoporosis) but because of chronic strain associated with trying to elevate the head in a person with a significant thoracic kyphosis.
Multiple vertebral fractures often are associated with psychological symptoms; this is not always appreciated. The changes in body configuration and back pain can lead to marked loss of self-image and a secondary depression. Altered balance, precipitated by the kyphosis and the anterior movement of the body’s center of gravity, leads to a fear of falling, a consequent tendency to remain indoors, and the onset of social isolation. These symptoms sometimes can be alleviated by family support and/or psychotherapy. Medication may be necessary when depressive features are present. Multiple thoracic vertebral fractures may be associated with restrictive lung disease symptoms and increased pulmonary infections. Multiple lumbar vertebral fractures are often associated with abdominal pain, constipation, protuberance, and early satiety. Multiple vertebral fractures are associated with greater age-specific mortality.
Multiple studies show that the majority of patients presenting in adulthood with fractures are not evaluated or treated for osteoporosis. Estimates suggest only about 20% of fracture patients receive follow-up care. Patients who sustain acute fractures are at dramatically elevated risk for more fractures, particularly within the first several years, and pharmacologic intervention can reduce that risk substantially. Recently, several studies have demonstrated the effectiveness of a relatively simple and inexpensive program that reduces the risk of subsequent fractures. In the Kaiser system, it is estimated that a 20% decline in hip fracture occurrence was seen with the introduction of what is called a fracture liaison service. This typically involves a health care professional (usually a nurse) whose job is to coordinate follow-up care and education of fracture patients. If the Kaiser experience can be repeated, there would be significant savings of health care dollars, as well as a dramatic drop in hip fracture incidence and a marked improvement in morbidity and mortality among the aging population.
MANAGEMENT OF THE UNDERLYING DISEASE
Patients presenting with typical osteoporosis-related fractures (certainly hip and spine) can be assumed to have osteoporosis and can be treated appropriately. Patients with osteoporosis by BMD are handled in a similar fashion. Other fracture patients and those with reduced bone mass can be classified according to their future risk of fracture and treated if that risk is sufficiently high. It must be emphasized, however, that risk assessment is an inexact science when applied to individual patients. Fractures are chance occurrences that can happen to anyone. Patients often do not understand the relative benefits of medications, compared to the perceived risks of the medications themselves.
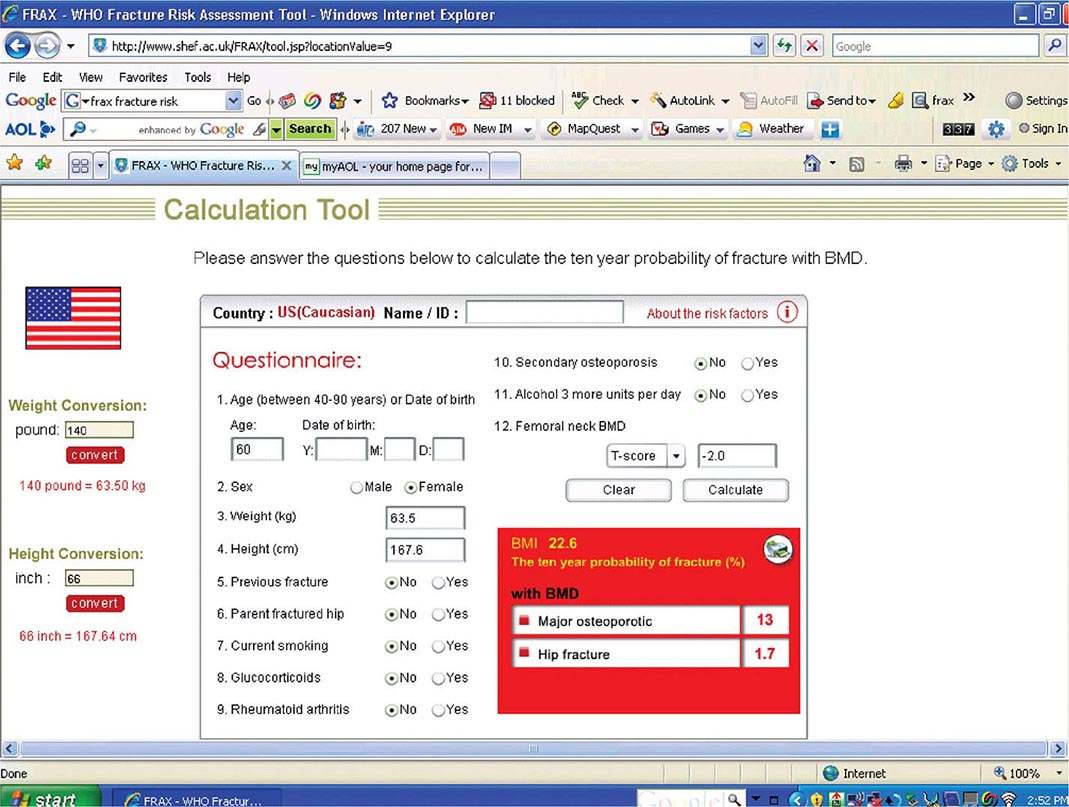
Risk Factor Reduction Several tools exist for risk assessment. The most commonly available is the FRAX tool, developed by a working party for the WHO, and available as part of the report from many DXA machines. It is also available online (http://www.shef.ac.uk/FRAX/tool.jsp?locationValue=9) (Fig. 425-7). In the United States, it has been estimated that it is cost-effective to treat a patient if the 10-year major fracture risk (including hip, clinical spine, proximal humerus, and tibia) from FRAX is ≥20% and/or the 10-year risk of hip fracture is ≥3%. FRAX is an imperfect tool because it does not include any assessment of fall risk and secondary causes are excluded when BMD is entered. Moreover, it does not include any term for multiple fractures or recent versus remote fracture. Nonetheless, it is useful as an educational tool for patients.
FIGURE 425-7 FRAX calculation tool. When the answers to the indicated questions are filled in, the calculator can be used to assess the 10-year probability of fracture. The calculator (available online at http://www.shef.ac.uk/FRAX/tool.jsp?locationValue=9) also can risk adjust for various ethnic groups.
After risk assessment, patients should be thoroughly educated to reduce the impact of modifiable risk factors associated with bone loss and falling. All medications that increase risk of falls, bone loss, or fractures should be reviewed to ensure that they are necessary and being used at the lowest required dose. For those on thyroid hormone replacement, TSH testing should be performed to confirm that an excessive dose is not being used, because biochemical and symptomatic thyrotoxicosis can be associated with increased bone loss. In patients who smoke, efforts should be made to facilitate smoking cessation. Reducing risk factors for falling also include alcohol abuse treatment and a review of the medical regimen for any drugs that might be associated with orthostatic hypotension and/or sedation, including hypnotics and anxiolytics. If nocturia occurs, the frequency should be reduced, if possible (e.g., by decreasing or modifying diuretic use), because arising in the middle of sleep is a common precipitant of a fall. Patients should be instructed about environmental safety with regard to eliminating exposed wires, curtain strings, slippery rugs, and mobile tables. Avoiding stocking feet on wood floors, checking carpet condition (particularly on stairs), and providing good light in paths to bathrooms and outside the home are important preventive measures. Treatment for impaired vision is recommended, particularly a problem with depth perception, which is specifically associated with increased falling risk. Elderly patients with neurologic impairment (e.g., stroke, Parkinson’s disease, Alzheimer’s disease) are particularly at risk of falling and require specialized supervision and care.
Nutritional Recommendations • CALCIUM A large body of data indicates that optimal calcium intake reduces bone loss and suppresses bone turnover. Recommended intakes from an Institute of Medicine report are shown in Table 425-5.
ADEQUATE CALCIUM INTAKE |
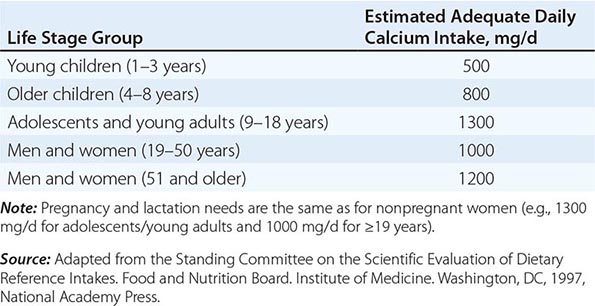
The National Health and Nutrition Examination Surveys (NHANES) have consistently documented that average calcium intakes fall considerably short of these recommendations. Food sources of calcium are dairy products (milk, yogurt, and cheese) and fortified foods such as certain cereals, waffles, snacks, juices, and crackers. Some of these fortified foods contain as much calcium per serving as milk. Green leafy vegetables and nuts, particularly almonds, are also sources of calcium, although their bioavailability may be lower than with dairy products. Calcium intake from the diet can also be assessed (Table 425-6) and calculators are available at NOF.org or NYSOPEP.org.
SIMPLE METHOD FOR CALCULATING DIETARY CALCIUM INTAKE |
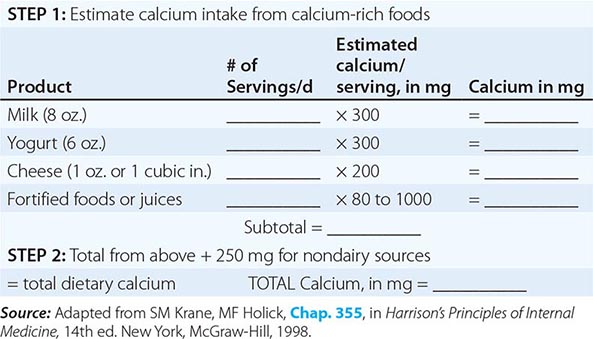
If a calcium supplement is required, it should be taken in doses sufficient to supplement dietary intake to bring total intake to the required level (1000–1200 mg/d). Doses of supplements should be ≤600 mg at a time, because the calcium absorption fraction decreases at higher doses. Calcium supplements should be calculated on the basis of the elemental calcium content of the supplement, not the weight of the calcium salt. Calcium supplements containing carbonate are best taken with food because they require acid for solubility. Calcium citrate supplements can be taken at any time. To confirm bioavailability, calcium supplements can be placed in distilled vinegar. They should dissolve within 30 min.
Several controlled clinical trials of calcium, mostly plus vitamin D, have confirmed reductions in clinical fractures, including fractures of the hip (~20–30% risk reduction). All recent studies of pharmacologic agents have been conducted in the context of calcium replacement (± vitamin D). Thus, it is standard practice to ensure an adequate calcium and vitamin D intake in patients with osteoporosis whether they are receiving additional pharmacologic therapy or not. A systematic review confirmed a greater BMD response to antiresorptive therapy when calcium intake was adequate.
Although side effects from supplemental calcium are minimal (eructation and constipation mostly with carbonate salts), individuals with a history of kidney stones should have a 24-h urine calcium determination before starting increased calcium to avoid significant hypercalciuria. Many studies confirm a small but significant increase in the risk of renal stones with calcium supplements, but not dietary calcium. A recent analysis of published data has suggested that high intakes of calcium from supplements are associated with an increase in the risk of heart disease. This is an evolving story with additional studies that confirm or refute this finding. Because high calcium supplement intakes increase the risk of renal stones and confer no extra benefit to the skeleton, the recommendation that total intakes should be between 1000 and 1200 mg/d is reasonable.
VITAMIN D Vitamin D is synthesized in skin under the influence of heat and ultraviolet light (Chap. 423). However, large segments of the population do not obtain sufficient vitamin D to maintain what is now considered an adequate supply [serum 25(OH)D consistently >75 μmol/L (30 ng/mL)]. Because vitamin D supplementation at doses that would achieve these serum levels is safe and inexpensive, the Institute of Medicine (based on obtaining a serum level of 20 ng/mL) recommends daily intakes of 200 IU for adults <50 years of age, 400 IU for those 50–70 years, and 600 IU for those >70 years. Multivitamin tablets usually contain 400 IU, and many calcium supplements also contain vitamin D. Some data suggest that higher doses (≥1000 IU) may be required in the elderly and chronically ill. The Institute of Medicine report suggests that it is safe to take up to 4000 IU/d. For those with osteoporosis or those at risk of osteoporosis, 1000–2000 IU/d can usually maintain serum 25(OH)D above 30 ng/mL.
OTHER NUTRIENTS Other nutrients such as salt, high animal protein intakes, and caffeine may have modest effects on calcium excretion or absorption. Adequate vitamin K status is required for optimal carboxylation of osteocalcin. States in which vitamin K nutrition or metabolism is impaired, such as with long-term warfarin therapy, have been associated with reduced bone mass. Research concerning cola intake is controversial but suggests a possible link to reduced bone mass through factors that are independent of caffeine. Although dark green leafy vegetables such as spinach and kale contain a fair amount of calcium, the high oxalate content reduces absorption of this calcium (but does not inhibit absorption of calcium from other food eaten simultaneously).
Magnesium is abundant in foods, and magnesium deficiency is quite rare in the absence of a serious chronic disease. Magnesium supplementation may be warranted in patients with inflammatory bowel disease, celiac disease, chemotherapy, severe diarrhea, malnutrition, or alcoholism. Dietary phytoestrogens, which are derived primarily from soy products and legumes (e.g., garbanzo beans [chickpeas] and lentils), exert some estrogenic activity but are insufficiently potent to justify their use in place of a pharmacologic agent in the treatment of osteoporosis.
Patients with hip fractures are often frail and relatively malnourished. Some data suggest an improved outcome in such patients when they are provided calorie and protein supplementation. Excessive protein intake can increase renal calcium excretion, but this can be corrected by an adequate calcium intake.
Exercise Exercise in young individuals increases the likelihood that they will attain the maximal genetically determined peak bone mass. Meta-analyses of studies performed in postmenopausal women indicate that weight-bearing exercise helps prevent bone loss but does not appear to result in substantial gain of bone mass. This beneficial effect wanes if exercise is discontinued. Most of the studies are short term, and a more substantial effect on bone mass is likely if exercise is continued over a long period. Exercise also has beneficial effects on neuromuscular function, and it improves coordination, balance, and strength, thereby reducing the risk of falling. A walking program is a practical way to start. Other activities, such as dancing, racquet sports, cross-country skiing, and use of gym equipment, are also recommended, depending on the patient’s personal preference and general condition. Even women who cannot walk benefit from swimming or water exercises, not so much for the effects on bone, which are quite minimal, but because of effects on muscle. Exercise habits should be consistent, optimally at least three times a week.
PHARMACOLOGIC THERAPIES
Before the mid-1990s, estrogen treatment, either by itself or in concert with a progestin, was the primary therapeutic agent for prevention or treatment of osteoporosis. There are now a number of new medications approved for osteoporosis and more under development. Some are agents that specifically treat osteoporosis (bisphosphonates, calcitonin, denosumab, and teriparatide [1-34hPTH]); others, such as selective estrogen response modulators (SERMs) and, most recently, an estrogen/SERM combination medication, have broader effects. The availability of these drugs allows therapy to be tailored to the needs of an individual patient.
Estrogens A large body of clinical trial data indicates that various types of estrogens (conjugated equine estrogens, estradiol, estrone, esterified estrogens, ethinyl estradiol, and mestranol) reduce bone turnover, prevent bone loss, and induce small increases in bone mass of the spine, hip, and total body. The effects of estrogen are seen in women with natural or surgical menopause and in late postmenopausal women with or without established osteoporosis. Estrogens are efficacious when administered orally or transdermally. For both oral and transdermal routes of administration, combined estrogen/progestin preparations are now available in many countries, obviating the problem of taking two tablets or using a patch and oral progestin.
Dose of Estrogen For oral estrogens, the standard recommended doses have been 0.3 mg/d for esterified estrogens, 0.625 mg/d for conjugated equine estrogens, and 5 μg/d for ethinyl estradiol. For transdermal estrogen, the commonly used dose supplies 50 μg estradiol per day, but a lower dose may be appropriate for some individuals. Dose-response data for conjugated equine estrogens indicate that lower doses (0.3 and 0.45 mg/d) are effective. Doses even lower have been associated with bone mass protection.
FRACTURE DATA Epidemiologic databases indicate that women who take estrogen replacement have a 50% reduction, on average, of osteoporotic fractures, including hip fractures. The beneficial effect of estrogen is greatest among those who start replacement early and continue the treatment; the benefit declines after discontinuation to the extent that there is no residual protective effect against fracture by 10 years after discontinuation. The first clinical trial evaluating fractures as secondary outcomes, the Heart and Estrogen-Progestin Replacement Study (HERS) trial, showed no effect of hormone therapy on hip or other clinical fractures in women with established coronary artery disease. These data made the results of the Women’s Health Initiative (WHI) exceedingly important (Chap. 413). The estrogen-progestin arm of the WHI in >16,000 postmenopausal healthy women indicated that hormone therapy reduces the risk of hip and clinical spine fracture by 34% and reduces the risk of all clinical fractures by 24%. There was similar antifracture efficacy seen with estrogen alone in women who had had a hysterectomy.
A few smaller clinical trials have evaluated spine fracture occurrence as an outcome with estrogen therapy. They have consistently shown that estrogen treatment reduces the incidence of vertebral compression fracture.
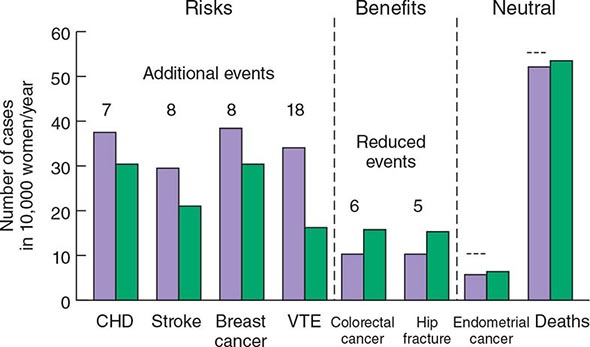
The WHI has provided a vast amount of data on the multisystemic effects of hormone therapy. Although earlier observational studies suggested that estrogen replacement might reduce heart disease, the WHI showed that combined estrogen-progestin treatment increased risk of fatal and nonfatal myocardial infarction by ~29%, confirming data from the HERS study. Other important relative risks included a 40% increase in stroke, a 100% increase in venous thromboembolic disease, and a 26% increase in risk of breast cancer. Subsequent analyses have confirmed the increased risk of stroke and, in a substudy, showed a twofold increase in dementia. Benefits other than the fracture reductions noted above included a 37% reduction in the risk of colon cancer. These relative risks have to be interpreted in light of absolute risk (Fig. 425-8). For example, out of 10,000 women treated with estrogen-progestin for 1 year, there will be 8 excess heart attacks, 8 excess breast cancers, 18 excess venous thromboembolic events, 5 fewer hip fractures, 44 fewer clinical fractures, and 6 fewer colorectal cancers. These numbers must be multiplied by years of hormone treatment. There was no effect of hormone treatment on the risk of uterine cancer or total mortality.
FIGURE 425-8 Effects of hormone therapy on event rates: green, placebo; purple, estrogen and progestin. CHD, coronary heart disease; VTE, venous thromboembolic events. (Adapted from Women’s Health Initiative. WHI HRT Update. Available at http://www.nhlbi.nih.gov/health/women/upd2002.htm.)
It is important to note that these WHI findings apply specifically to hormone treatment in the form of conjugated equine estrogen plus medroxyprogesterone acetate. The relative benefits and risks of unopposed estrogen in women who had hysterectomies vary somewhat. They still show benefits against fracture occurrence and increased risk of venous thrombosis and stroke, similar in magnitude to the risks for combined hormone therapy. In contrast, though, the estrogen-only arm of WHI indicated no increased risk of heart attack or breast cancer. The data suggest that at least some of the detrimental effects of combined therapy are related to the progestin component. In addition, there is the possibility, suggested by primate data, that the risk accrues mainly to women who have some years of estrogen deficiency before initiating treatment. (The average woman in the WHI was more than 10 years from the last menstrual period). Nonetheless, there is reluctance among women to use estrogen/hormone therapy, and the U.S. Preventive Services Task Force has specifically suggested that estrogen/hormone therapy not be used for disease prevention.
MODE OF ACTION Two subtypes of ERs, α and β, have been identified in bone and other tissues. Cells of monocyte lineage express both ERα and ERβ, as do osteoblasts. Estrogen-mediated effects vary with the receptor type. Using ER knockout mouse models, elimination of ERα produces a modest reduction in bone mass, whereas mutation of ERβ has less of an effect on bone. A male patient with a homozygous mutation of ERα had markedly decreased bone density as well as abnormalities in epiphyseal closure, confirming the important role of ERα in bone biology. The mechanism of estrogen action in bone is an area of active investigation (Fig. 425-5). Although data are conflicting, estrogens may inhibit osteoclasts directly. However, the majority of estrogen (and androgen) effects on bone resorption are mediated through paracrine factors produced by osteoblasts and osteocytes. These actions include decreasing RANKL production and increasing OPG production by osteoblasts.
Progestins In women with a uterus, daily progestin or cyclical progestins at least 12 days per month are prescribed in combination with estrogens to reduce the risk of uterine cancer. Medroxyprogesterone acetate and norethindrone acetate blunt the high-density lipoprotein response to estrogen, but micronized progesterone does not. Neither medroxyprogesterone acetate nor micronized progesterone appears to have an independent effect on bone; at lower doses of estrogen, norethindrone acetate may have an additive benefit. On breast tissue, progestins may increase the risk of breast cancer.
SERMs
Two SERMs are used currently in postmenopausal women: raloxifene, which is approved for the prevention and treatment of osteoporosis as well as the prevention of breast cancer, and tamoxifen, which is approved for the prevention and treatment of breast cancer. A third SERM, bazedoxifene, has been complexed with conjugated estrogen, creating a tissue selective estrogen complex (TSEC). This agent has been approved for prevention of osteoporosis.
Tamoxifen reduces bone turnover and bone loss in postmenopausal women compared with placebo groups. These findings support the concept that tamoxifen acts as an estrogenic agent in bone. There are limited data on the effect of tamoxifen on fracture risk, but the Breast Cancer Prevention Study indicated a possible reduction in clinical vertebral, hip, and Colles’ fractures. The major benefit of tamoxifen is on breast cancer occurrence. The breast cancer prevention trial indicated that tamoxifen administration over 4–5 years reduced the incidence of new invasive and noninvasive breast cancer by ~45% in women at increased risk of breast cancer. The incidence of ER-positive breast cancers was reduced by 65%. Tamoxifen increases the risk of uterine cancer and increases risk of venous thrombosis, cataracts, and possibly stroke in postmenopausal women, limiting its use for breast cancer prevention in women at low or moderate risk.
Raloxifene (60 mg/d) has effects on bone turnover and bone mass that are very similar to those of tamoxifen, indicating that this agent is also estrogenic on the skeleton. The effect of raloxifene on bone density (+1.4–2.8% vs placebo in the spine, hip, and total body) is somewhat less than that seen with standard doses of estrogens. Raloxifene reduces the occurrence of vertebral fracture by 30–50%, depending on the population; however, there are no data confirming that raloxifene can reduce the risk of nonvertebral fractures over 8 years of observation.
Raloxifene, like tamoxifen and estrogen, has effects in other organ systems. The most beneficial effect appears to be a reduction in invasive breast cancer (mainly decreased ER-positive) occurrence of ~65% in women who take raloxifene compared to placebo. In a head-to-head study, raloxifene was as effective as tamoxifen in preventing breast cancer in high-risk women, and raloxifene is now FDA approved for this indication. In a further study, raloxifene had no effect on heart disease in women with increased risk for this outcome. In contrast to tamoxifen, raloxifene is not associated with an increase in the risk of uterine cancer or benign uterine disease. Raloxifene increases the occurrence of hot flashes but reduces serum total and low-density lipoprotein cholesterol, lipoprotein(a), and fibrinogen. Raloxifene, with positive effects on breast cancer and vertebral fractures, has become a useful agent for the treatment of the younger asymptomatic postmenopausal woman. In some women, a recurrence of menopausal hot flashes may occur. Usually this is evanescent, but occasionally, it is sufficiently impactful on daily life and sleep that the drug must be withdrawn. Raloxifene increases the risk of deep vein thrombosis and may increase the risk of death from stroke among older women. Consequently, it is not usually recommended for women over 70 years of age.
The main advantage of the bazedoxifene/conjugated estrogen compound is that the bazedoxifene protects uterine tissue from the effects of estrogen and makes it possible to avoid taking a progestin, while using an estrogen primarily for control of menopausal symptoms. The TSEC prevents bone loss somewhat more potently than raloxifene alone and appears safe for the breast.
MODE OF ACTION OF SERMS All SERMs bind to the ER, but each agent produces a unique receptor-drug conformation. As a result, specific co-activator or co-repressor proteins are bound to the receptor (Chap. 400e), resulting in differential effects on gene transcription that vary depending on other transcription factors present in the cell. Another aspect of selectivity is the affinity of each SERM for the different ERα and ERβ subtypes, which are expressed differentially in various tissues. These tissue-selective effects of SERMs offer the possibility of tailoring estrogen therapy to best meet the needs and risk factor profile of an individual patient.
Bisphosphonates Alendronate, risedronate, ibandronate, and zoledronic acid are approved for the prevention and treatment of postmenopausal osteoporosis. Alendronate, risedronate, and zoledronic acid are also approved for the treatment of steroid-induced osteoporosis, and risedronate and zoledronic acid are approved for prevention of steroid-induced osteoporosis. Alendronate, risedronate, and zoledronic acid are approved for treatment of osteoporosis in men.
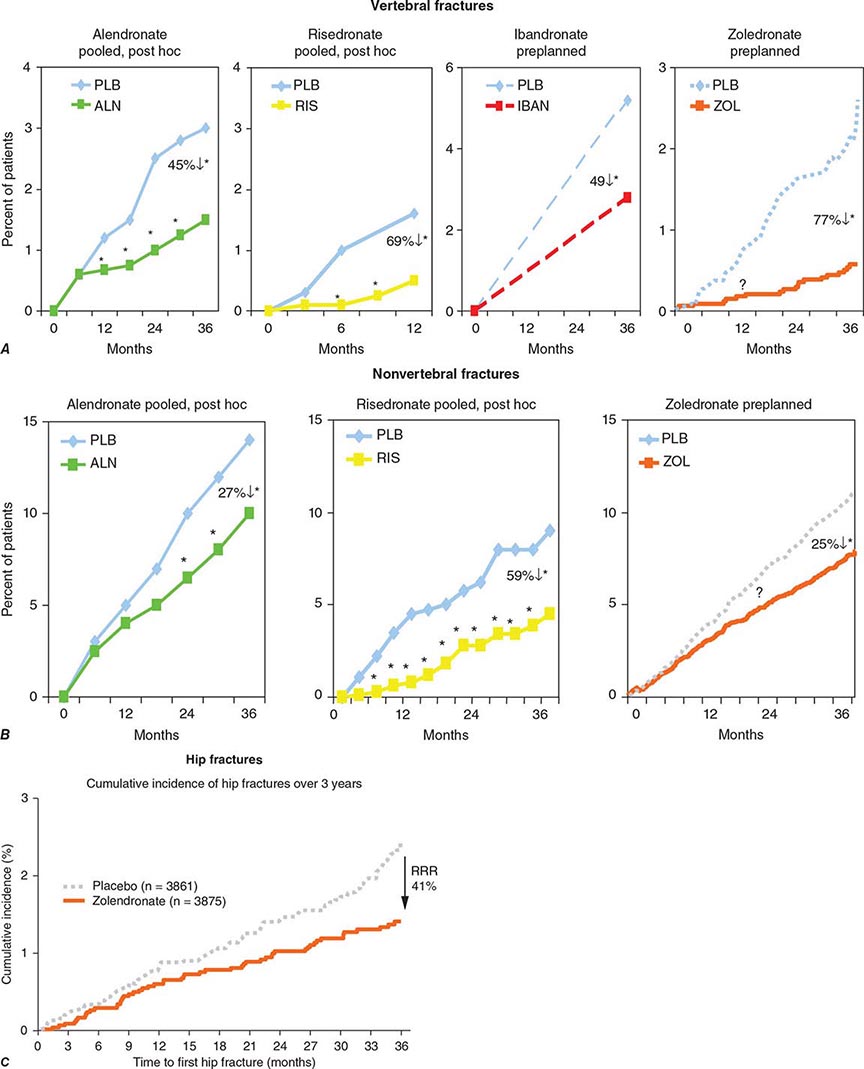
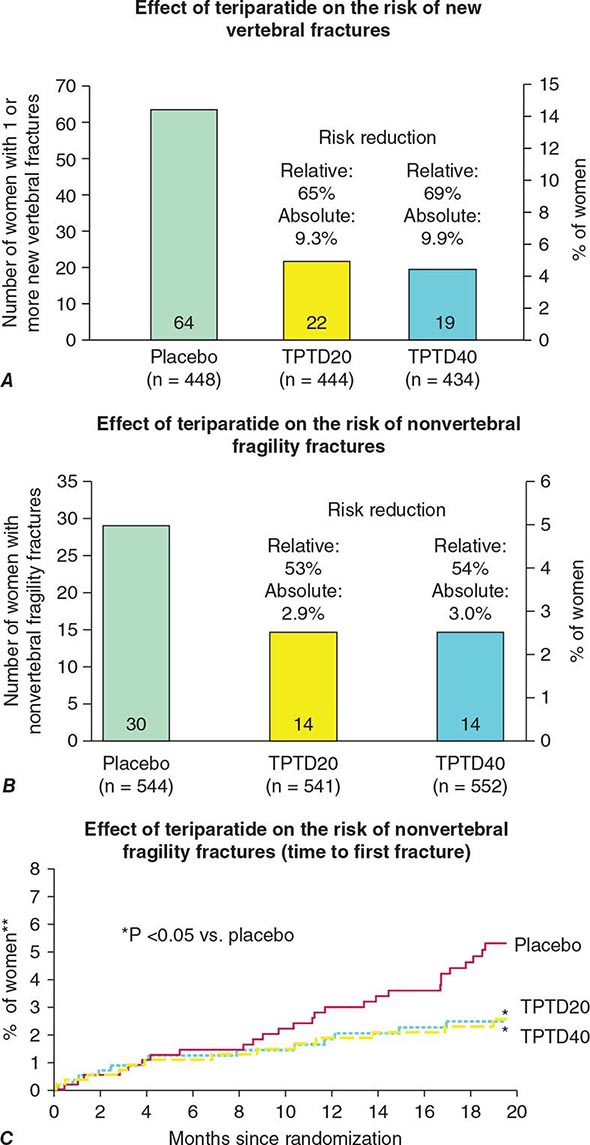
Alendronate has been shown to decrease bone turnover and increase bone mass in the spine by up to 8% versus placebo and by 6% versus placebo in the hip. Multiple trials have evaluated its effect on fracture occurrence. The Fracture Intervention Trial provided evidence in >2000 women with prevalent vertebral fractures that daily alendronate treatment (5 mg/d for 2 years and 10 mg/d for 9 months afterward) reduces vertebral fracture risk by about 50%, multiple vertebral fractures by up to 90%, and hip fractures by up to 50%. Several subsequent trials have confirmed these findings (Fig. 425-9). For example, in a study of >1900 women with low bone mass treated with alendronate (10 mg/d) versus placebo, the incidence of all nonvertebral fractures was reduced by ~47% after only 1 year. In the United States, the 10-mg dose is approved for treatment of osteoporosis and 5 mg/d is used for prevention.
FIGURE 425-9 Effects of various bisphosphonates on clinical vertebral fractures (A), nonvertebral fractures (B), and hip fractures (C). PLB, placebo; RRR, relative risk reduction. (After DM Black et al: J Clin Endocrinol Metab 85:4118, 2000; C Roux et al: Curr Med Res Opin 4:433, 2004; CH Chesnut et al: J Bone Miner Res 19:1241, 2004; DM Black et al: N Engl J Med 356:1809, 2007; JT Harrington et al: Calcif Tissue Int 74:129, 2003.)
Trials comparing once-weekly alendronate, 70 mg, with daily 10-mg dosing have shown equivalence with regard to bone mass and bone turnover responses. Consequently, once-weekly therapy generally is preferred because of the low incidence of gastrointestinal side effects and ease of administration. Alendronate should be given with a full glass of water before breakfast, because bisphosphonates are poorly absorbed. Because of the potential for esophageal irritation, alendronate is contraindicated in patients who have stricture or inadequate emptying of the esophagus. It is recommended that patients remain upright for at least 30 min after taking the medication to avoid esophageal irritation. Cases of esophagitis, esophageal ulcer, and esophageal stricture have been described, but the incidence appears to be low. In clinical trials, overall gastrointestinal symptomatology was no different with alendronate than with placebo. Alendronate is also available in a preparation that contains vitamin D.
Risedronate also reduces bone turnover and increases bone mass. Controlled clinical trials have demonstrated 40–50% reduction in vertebral fracture risk over 3 years, accompanied by a 40% reduction in clinical nonspine fractures. The only clinical trial specifically designed to evaluate hip fracture outcome (HIP) indicated that risedronate reduced hip fracture risk in women in their seventies with confirmed osteoporosis by 40%. In contrast, risedronate was not effective at reducing hip fracture occurrence in older women (80+ years) without proven osteoporosis. Studies have shown that 35 mg of risedronate administered once weekly is therapeutically equivalent to 5 mg/d and that 150 mg once monthly is therapeutically equivalent to 35 mg once weekly. Patients should take risedronate with a full glass of plain water to facilitate delivery to the stomach and should not lie down for 30 min after taking the drug. The incidence of gastrointestinal side effects in trials with risedronate was similar to that of placebo. A new preparation, which allows risedronate to be taken with food, was recently approved.
Etidronate was the first bisphosphonate to be approved, initially for use in Paget’s disease and hypercalcemia. This agent has also been used in osteoporosis trials of smaller magnitude than those performed for alendronate and risedronate but is not approved by the FDA for treatment of osteoporosis. Etidronate probably has some efficacy against vertebral fracture when given as an intermittent cyclical regimen (2 weeks on, 2.5 months off). Its effectiveness against nonvertebral fractures has not been studied.
Ibandronate is the third amino-bisphosphonate approved in the United States. Ibandronate (2.5 mg/d) has been shown in clinical trials to reduce vertebral fracture risk by ~40% but with no overall effect on nonvertebral fractures. In a post hoc analysis of subjects with a femoral neck T-score of –3 or below, ibandronate reduced the risk of nonvertebral fractures by ~60%. In clinical trials, ibandronate doses of 150 mg/month PO or 3 mg every 3 months IV had greater effects on turnover and bone mass than did 2.5 mg/d. Patients should take oral ibandronate in the same way as other bisphosphonates, but with 1 h elapsing before other food or drink (other than plain water).
Zoledronic acid is a potent bisphosphonate with a unique administration regimen (5 mg by slow IV infusion annually). The data confirm that it is highly effective in fracture risk reduction. In a study of >7000 women followed for 3 years, zoledronic acid (three annual infusions) reduced the risk of vertebral fractures by 70%, nonvertebral fractures by 25%, and hip fractures by 40%. These results were associated with less height loss and disability. In the treated population, there was an increased risk of transient postdose symptoms (acute-phase reaction) manifested by fever, arthralgia, myalgias, and headache. The symptoms usually last less than 48 h. An increased risk of atrial fibrillation and transient but not permanent reduction in renal function was seen in comparison to placebo. Detailed evaluation of all bisphosphonates failed to confirm that these agents increased the risk of atrial fibrillation. Zoledronic acid is the only osteoporosis agent that has been studied in the elderly with a prior hip fracture. The risk of all clinical fractures was reduced significantly by about 35%, and there was a trend toward reduced risk of a second hip fracture (effect size similar to that seen above). There was also a reduction in mortality of about 30% that was not completely accounted for the reduced hip fracture risk.
Recently there has been concern about two potential side effects associated with bisphosphonate use. The first is osteonecrosis of the jaw (ONJ). ONJ usually follows a dental procedure in which bone is exposed (extractions or dental implants). It is presumed that the exposed bone becomes infected and dies. It is not uncommon among cancer victims with multiple myeloma or patients receiving high doses of bisphosphonates for skeletal metastases, but is rare among persons with osteoporosis on usual doses of bisphosphonates. The second side effect is called atypical femur fracture. These are unusual fractures that occur distal to the lesser trochanter and anywhere along the femoral shaft. They are often preceded by pain in the lateral thigh or groin that can be present for weeks or months before the fracture. The fractures occur with trivial trauma, sometimes completely spontaneously, and are primarily transverse, with a medial break when complete and minimally comminuted. A localized periosteal reaction, consistent with a stress fracture, is often seen in the lateral cortex (Fig. 425-10). The overall risk is low (suggested to be about one-one hundredth to one-tenth that of hip fracture) but appears to increase in incidence with long-term use of bisphosphonates. Although the fractures may be bisphosphonate related in many individuals, they clearly occur in patients with no prior bisphosphonate exposure. When complete, they require surgical fixation and may be difficult to heal. Anabolic medication may accelerate healing of these fractures in some patients, and surgery can sometimes be avoided. Patients initiating bisphosphonates need to be warned that if they develop thigh or groin pain they must notify their physician. Routine x-rays will sometimes pick up cortical thickening or even a stress fracture, but more commonly MRI or technetium bone scan is required. The presence of an abnormality requires at minimum a period of modified weight bearing and may need prophylactic rodding of the femur. It is important to realize that these fractures may be bilateral, and when an abnormality is found, the other femur should be investigated.
FIGURE 425-10 An atypical femur fracture (AFF) of the femoral diaphysis. A. Note the transverse fracture line in the lateral cortex that becomes oblique as it progresses medially across the femur (white arrow). B. On radiograph obtained immediately after intramedullary rod placement, a small area of periosteal thickening of the lateral cortex is visible (white arrow). C. On radiograph obtained at 6 weeks, note callus formation of the fracture site (white arrow). D. On radiograph obtained at 3 months, there is a mature callus that has failed to bridge the cortical gap (white arrow). Note the localized periosteal and/or endosteal thickening of the lateral cortex at the fracture site (white arrow). (From E Shane et al: J Bone Min Res 29:1-23, 2014. Courtesy of Fergus McKiernan.)
MODE OF ACTION Bisphosphonates are structurally related to pyrophosphates, compounds that are incorporated into bone matrix. Bisphosphonates specifically impair osteoclast function and reduce osteoclast number, in part by inducing apoptosis. Recent evidence suggests that the nitrogen-containing bisphosphonates also inhibit protein prenylation, one of the end products in the mevalonic acid pathway, by inhibiting the enzyme farnesyl pyrophosphate synthase. This effect disrupts intracellular protein trafficking and ultimately may lead to apoptosis. Some bisphosphonates have very long retention in the skeleton and may exert long-term effects. The consequences of this, if any, are unknown.
Calcitonin Calcitonin is a polypeptide hormone produced by the thyroid gland (Chap. 424). Its physiologic role is unclear because no skeletal disease has been described in association with calcitonin deficiency or excess. Calcitonin preparations are approved by the FDA for Paget’s disease, hypercalcemia, and osteoporosis in women >5 years past menopause. Concerns have been raised about an increase in the incidence of cancer associated with calcitonin use. Initially, the cancer noted was of the prostate, but an analysis of all data suggested a more general increase in cancer risk. In Europe, the European Medicines Agency (EMA) has removed the osteoporosis indication, and an FDA Advisory Committee has voted for a similar change in the United States.
Injectable calcitonin produces small increments in bone mass of the lumbar spine. However, difficulty of administration and frequent reactions, including nausea and facial flushing, make general use limited. A nasal spray containing calcitonin (200 IU/d) is available for treatment of osteoporosis in postmenopausal women. One study suggests that nasal calcitonin produces small increments in bone mass and a small reduction in new vertebral fractures in calcitonin-treated patients versus those on calcium alone. There has been no proven effectiveness against nonvertebral fractures.
Calcitonin is not indicated for prevention of osteoporosis and is not sufficiently potent to prevent bone loss in early postmenopausal women. Calcitonin might have an analgesic effect on bone pain, both in the subcutaneous and possibly the nasal form.
MODE OF ACTION Calcitonin suppresses osteoclast activity by direct action on the osteoclast calcitonin receptor. Osteoclasts exposed to calcitonin cannot maintain their active ruffled border, which normally maintains close contact with underlying bone.
Denosumab A novel agent that was given twice yearly by SC administration in a randomized controlled trial in postmenopausal women with osteoporosis has been shown to increase BMD in the spine, hip, and forearm and reduce vertebral, hip, and nonvertebral fractures over a 3-year period by 70, 40, and 20%, respectively (Fig. 425-11). Other clinical trials indicate ability to increase bone mass in postmenopausal women with low bone mass (above osteoporosis range) and in postmenopausal women with breast cancer treated with hormonal agents. Furthermore, a study of men with prostate cancer treated with gonadotropin-releasing hormone (GnRH) agonist therapy indicated the ability of denosumab to improve bone mass and reduce vertebral fracture occurrence. Denosumab was approved by the FDA in 2010 for the treatment of postmenopausal women who have a high risk for osteoporotic fractures, including those with a history of fracture or multiple risk factors for fracture, and those who have failed or are intolerant to other osteoporosis therapy. Denosumab is also approved for the treatment of osteoporosis in men at high risk, men with prostate cancer on GnRH agonist therapy, and women with breast cancer on aromatase inhibitor therapy.
FIGURE 425-11 Effects of denosumab on new vertebral fractures (A) and times to nonvertebral and hip fracture (B and C). RR, relative risk. (After SR Cummings et al: N Engl J Med 361:756, 2009.)
MODE OF ACTION Denosumab is a fully human monoclonal antibody to RANKL, the final common effector of osteoclast formation, activity, and survival. Denosumab binds to RANKL, inhibiting its ability to initiate formation of mature osteoclasts from osteoclast precursors and to bring mature osteoclasts to the bone surface and initiate bone resorption. Denosumab also plays a role in reducing the survival of the osteoclast. Through these actions on the osteoclast, denosumab induces potent antiresorptive action, as assessed biochemically and histomorphometrically, and may contribute to the occurrence of ONJ. Atypical femur fractures have also been noted. Serious adverse reactions include hypocalcemia, skin infections (usually cellulitis of the lower extremity), and dermatologic reactions such as dermatitis, rashes, and eczema. The effects of denosumab are rapidly reversible. If denosumab is stopped, bone will be lost rapidly if another agent is not used.
Parathyroid Hormone Endogenous PTH is an 84-amino-acid peptide that is largely responsible for calcium homeostasis (Chap. 424). Although chronic elevation of PTH, as occurs in hyperparathyroidism, is associated with bone loss (particularly cortical bone), PTH when given exogenously as a daily injection exerts anabolic effects on bone. Teriparatide (1-34hPTH) is approved for the treatment of osteoporosis in both men and women at high risk for fracture. In a pivotal study (median time of treatment, 19 months’ duration), 20 μg of teriparatide daily by SC injection reduced vertebral fractures by 65% and nonvertebral fractures by 45% (Fig. 425-12). Treatment is administered as a single daily injection given for a maximum of 2 years. Teriparatide produces increases in bone mass and mediates architectural improvements in skeletal structure. These effects are lower when patients have been exposed previously to bisphosphonates, possibly in proportion to the potency of the antiresorptive effect. When teriparatide is being considered for treatment-naive patients, it is best administered as monotherapy and followed by an antiresorptive agent such as a bisphosphonate. If teriparatide treatment is not followed by an antiresorptive agent, the bone gained is rapidly lost.
FIGURE 425-12 Effects of teriparatide (TPTD) on new vertebral fractures (A) and nonvertebral fragility fractures (B and C). (After RM Neer et al: N Engl J Med 344:1434, 2001.)
Side effects of teriparatide are generally mild and can include leg cramps, muscle pain, weakness, dizziness, headache, and nausea. Rodents given prolonged treatment with PTH in relatively high doses developed osteogenic sarcomas. Long-term surveillance studies suggest no association between 2 years of teriparatide administration and osteosarcoma risk in humans.
PTH use may be limited by its mode of administration; alternative modes of delivery are being investigated. The optimal frequency of administration also remains to be established, and it is possible that PTH might be effective when used intermittently. Cost also may be a limiting factor. In some settings, the effect of PTH might be enhanced by combination with an antiresorptive agent. This might be particularly important in patients who have been treated previously with bisphosphonate medications.
MODE OF ACTION Exogenously administered PTH appears to have direct actions on osteoblast activity, with biochemical and histomorphometric evidence of de novo bone formation early in response to PTH, before activation of bone resorption. Subsequently, PTH activates bone remodeling but still appears to favor bone formation over bone resorption. PTH stimulates Wnt signaling, IGF-I, and collagen production and appears to increase osteoblast number by stimulating replication, enhancing osteoblast recruitment, and inhibiting apoptosis. Unlike all other treatments, PTH produces a true increase in bone tissue and an apparent restoration of bone microarchitecture (Fig. 425-13).
FIGURE 425-13 Effect of parathyroid hormone (PTH) treatment on bone microarchitecture. Paired biopsy specimens from a 64-year-old woman before (A) and after (B) treatment with PTH. (From DW Dempster et al: J Bone Miner Res 16:1846, 2001.)
Fluoride Fluoride has been available for many years and is a potent stimulator of osteoprogenitor cells when studied in vitro. It has been used in multiple osteoporosis studies with conflicting results, in part because of the use of varying doses and preparations. Despite increments in bone mass of up to 10%, there are no consistent effects of fluoride on vertebral or nonvertebral fracture; the latter may actually increase when high doses of fluoride are used. Fluoride remains an experimental agent despite its long history and multiple studies.
Strontium Ranelate Strontium ranelate is approved in several European countries for the treatment of osteoporosis. It increases bone mass throughout the skeleton; in clinical trials, the drug reduced the risk of vertebral fractures by 37% and that of nonvertebral fractures by 14%. It appears to be modestly antiresorptive while at the same time not causing as much of a decrease in bone formation (measured biochemically). Strontium is incorporated into hydroxyapatite, replacing calcium, a feature that might explain some of its fracture benefits. Small increased risks of venous thrombosis, sometimes severe dermatologic reactions, seizures, and abnormal cognition have been seen and require further study. An increase in risk of cardiovascular disease has also been associated with use of strontium, such that the EMA has restricted its use at present.
Other Potential Anabolic Agents Several small studies of growth hormone (GH), alone or in combination with other agents, have not shown consistent or substantial positive effects on skeletal mass. Many of these studies have been relatively short term, and the effects of GH, growth hormone–releasing hormone, and the IGFs are still under investigation. Anabolic steroids, mostly derivatives of testosterone, act primarily as antiresorptive agents to reduce bone turnover but also may stimulate osteoblastic activity. Effects on bone mass remain unclear but appear weak in general, and use is limited by masculinizing side effects. Several observational studies suggested that the statin drugs, used to treat hypercholesterolemia, may be associated with increased bone mass and reduced fractures, but conclusions from clinical trials have been largely negative. Early studies with sclerostin antibodies, which inhibit sclerostin, activate Wnt, and might be highly anabolic to bone, are under development. Odanacatib is a mixed antiresorptive, partial bone formation stimulator that is currently in the late stages of development.
NONPHARMACOLOGIC APPROACHES
In some early studies, protective pads worn around the outer thigh, which cover the trochanteric region of the hip, were able to prevent hip fractures in elderly residents in nursing homes. Randomized controlled trials of hip protectors have been unable to confirm these early findings. Therefore, the efficacy of hip protectors remains controversial at this time.
Kyphoplasty and vertebroplasty are also useful nonpharmacologic approaches for the treatment of painful vertebral fractures. However, no long-term data are available.
TREATMENT MONITORING
There are currently no well-accepted guidelines for monitoring treatment of osteoporosis. Because most osteoporosis treatments produce small or moderate bone mass increments on average, it is reasonable to consider BMD as a monitoring tool. Changes must exceed ~4% in the spine and 6% in the hip to be considered significant in any individual. The hip is the preferred site due to larger surface area and greater reproducibility. Medication-induced increments may require several years to produce changes of this magnitude (if they do at all). Consequently, it can be argued that BMD should be repeated at intervals >2 years. Only significant BMD reductions should prompt a change in medical regimen, because it is expected that many individuals will not show responses greater than the detection limits of the current measurement techniques.
Biochemical markers of bone turnover may prove useful for treatment monitoring, but little hard evidence currently supports this concept; it remains unclear which endpoint is most useful. If bone turnover markers are used, a determination should be made before therapy is started and repeated ≥4 months after therapy is initiated. In general, a change in bone turnover markers must be 30–40% lower than the baseline to be significant because of the biologic and technical variability in these tests. A positive change in biochemical markers and/or bone density can be useful to help patients adhere to treatment regimens.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree