INTRODUCTION
Viral hepatitis is a systemic disease primarily involving the liver. Most cases of acute viral hepatitis in children and adults are caused by one of the following five agents: hepatitis A virus (HAV), the etiologic agent of viral hepatitis type A (infectious hepatitis); hepatitis B virus (HBV), which is associated with viral hepatitis B (serum hepatitis); hepatitis C virus (HCV), the agent of hepatitis C (common cause of posttransfusion hepatitis); hepatitis D (HDV), a defective virus dependent on coinfection with HBV; or hepatitis E virus (HEV), the agent of enterically transmitted hepatitis. Additional well-characterized viruses that can cause sporadic hepatitis, such as yellow fever virus, cytomegalovirus, Epstein-Barr virus, herpes simplex virus, rubella virus, and the enteroviruses, are discussed in other chapters. Hepatitis viruses produce acute inflammation of the liver, resulting in a clinical illness characterized by fever, gastrointestinal symptoms such as nausea and vomiting, and jaundice. Regardless of the virus type, identical histopathologic lesions are observed in the liver during acute disease.
PROPERTIES OF HEPATITIS VIRUSES
The characteristics of the five known hepatitis viruses are shown in Table 35-1. Nomenclature of the hepatitis viruses, antigens, and antibodies is presented in Table 35-2.
| Virus | Hepatitis A | Hepatitis B | Hepatitis C | Hepatitis D | Hepatitis E |
|---|---|---|---|---|---|
| Family | Picornaviridae | Hepadnaviridae | Flaviviridae | Unclassified | Hepeviridae |
| Genus | Hepatovirus | Orthohepadnavirus | Hepacivirus | Deltavirus | Hepevirus |
| Virion | 27 nm, icosahedral | 42 nm, spherical | 60 nm, spherical | 35 nm, spherical | 30–32 nm, icosahedral |
| Envelope | No | Yes (HBsAg) | Yes | Yes (HBsAg) | No |
| Genome | ssRNA | dsDNA | ssRNA | ssRNA | ssRNA |
| Genome size (kb) | 7.5 | 3.2 | 9.4 | 1.7 | 7.2 |
| Stability | Heat and acid stable | Acid sensitive | Ether sensitive, acid sensitive | Acid sensitive | Heat stable |
| Transmission | Fecal–oral | Parenteral | Parenteral | Parenteral | Fecal–oral |
| Prevalence | High | High | Moderate | Low, regional | Regional |
| Fulminant disease | Rare | Rare | Rare | Frequent | In pregnancy |
| Chronic disease | Never | Often | Often | Often | Never |
| Oncogenic | No | Yes | Yes | Unknown | No |
| Disease | Component of System | Definition |
|---|---|---|
| Hepatitis A | HAV | Hepatitis A virus. Etiologic agent of infectious hepatitis. A picornavirus, the prototype of genus Hepatovirus |
| Anti-HAV | Antibody to HAV. Detectable at onset of symptoms; lifetime persistence | |
| IgM anti-HAV | IgM class antibody to HAV. Indicates recent infection with hepatitis A; positive result up to 4–6 months after infection | |
| Hepatitis B | HBV | Hepatitis B virus. Etiologic agent of serum hepatitis. A hepadnavirus |
| HBsAg | Hepatitis B surface antigen. Surface antigen(s) of HBV detectable in large quantity in serum; several subtypes identified | |
| HBeAg | Hepatitis B e antigen. Associated with HBV nucleocapsid; indicates viral replication; circulates as soluble antigen in serum | |
| HBcAg | Hepatitis B core antigen | |
| Anti-HBs | Antibody to HBsAg. Indicates past infection with and immunity to HBV, presence of passive antibody from HBIG, or immune response from HBV vaccine | |
| Anti-HBe | Antibody to HBeAg. Presence in serum of HBV carrier suggests lower titer of HBV | |
| Anti-HBc | Antibody to HBcAg. Indicates infection with HBV at some undefined time in the past | |
| IgM anti-HBc | IgM class antibody to HBcAg. Indicates recent infection with HBV; positive result for 4–6 months after infection | |
| Hepatitis C | HCV | Hepatitis C virus, a common etiologic agent of posttransfusion hepatitis. A flavivirus, genus Hepacivirus |
| Anti-HCV | Antibody to HCV | |
| Hepatitis D | HDV | Hepatitis D virus. Etiologic agent of delta hepatitis; causes infection only in presence of HBV |
| HDAg | Delta antigen (delta-Ag). Detectable in early acute HDV infection | |
| Anti-HD | Antibody to delta-Ag (anti-delta). Indicates past or present infection with HDV | |
| Hepatitis E | HEV | Hepatitis E virus. Enterically transmitted hepatitis virus. Causes large epidemics in Asia, North and West Africa, and Mexico; fecal–oral or waterborne transmission. A hepevirus |
| Immune globulins | IG | Immune globulin USP. Contains antibodies to HAV; no antibodies to HBsAg, HCV, or human immunodeficiency virus |
| HBIG | Hepatitis B immune globulin. Contains high titers of antibodies to HBV |
HAV is a distinct member of the picornavirus family (see Chapter 36). HAV is a 27- to 32-nm spherical particle with cubic symmetry containing a linear single-stranded RNA genome with a size of 7.5 kb. It is assigned to picornavirus genus, Hepatovirus. Only one serotype is known. There is no antigenic cross-reactivity with the other hepatitis viruses. Genomic sequence analysis of a variable region involving the junction of the 1D and 2A genes divided HAV isolates into seven genotypes. Important properties of the family Picornaviridae are listed in Table 36-1.
HAV is stable to treatment with 20% ether, acid (pH 1.0 for 2 hours), and heat (60°C for 1 hour), and its infectivity can be preserved for at least 1 month after being dried and stored at 25°C or for years at −20°C. The virus is destroyed by autoclaving (121°C for 20 minutes), boiling in water for 5 minutes, dry heat (180°C for 1 hour), ultraviolet irradiation (1 minute at 1.1 watts), treatment with formalin (1:4000 for 3 days at 37°C), or treatment with chlorine (10–15 ppm for 30 minutes). Heating food to above 85°C (185°F) for 1 minute and disinfecting surfaces with sodium hypochlorite (1:100 dilution of chlorine bleach) are necessary to inactivate HAV. The relative resistance of HAV to disinfection procedures emphasizes the need for extra precautions in dealing with hepatitis patients and their products.
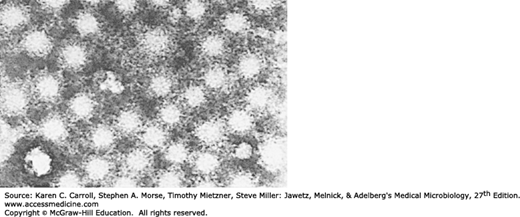
HAV initially was identified in stool and liver preparations by using immune electron microscopy (Figure 35-1). Sensitive serologic assays and polymerase chain reaction (PCR) methods have made it possible to detect HAV in stools and other samples and to measure specific antibody in serum.
Various primate cell lines will support growth of HAV, although fresh isolates of virus are difficult to adapt and grow. Usually, no cytopathic effects are apparent. Mutations in the viral genome are selected during adaptation to tissue culture.
HBV is classified as a hepadnavirus (Table 35-3). HBV establishes chronic infections, especially in those infected as infants; it is a major factor in the eventual development of liver disease and hepatocellular carcinoma in those individuals.
| Virion: About 42 nm in diameter overall (nucleocapsids, 18 nm). |
| Genome: One molecule of double-stranded DNA, circular, 3.2 kbp. In virion, negative DNA strand is full length, and positive DNA strand is partially complete. The gap must be completed at the beginning of the replication cycle. |
| Proteins: Two major polypeptides (one glycosylated) are present in HBsAg; one polypeptide is present in HBcAg. |
| Envelope: Contains HBsAg and lipid. |
| Replication: By means of an intermediate RNA copy of the DNA genome (HBcAg in nucleus; HBsAg in cytoplasm). Both mature virus and 22-nm spherical particles consist of HBsAg secreted from the cell surface. |
| Outstanding characteristics: |
| Family is made up of many types that infect humans and lower animals (eg, woodchucks, squirrels, ducks). |
| Cause acute and chronic hepatitis, often progressing to permanent carrier states and hepatocellular carcinoma. |
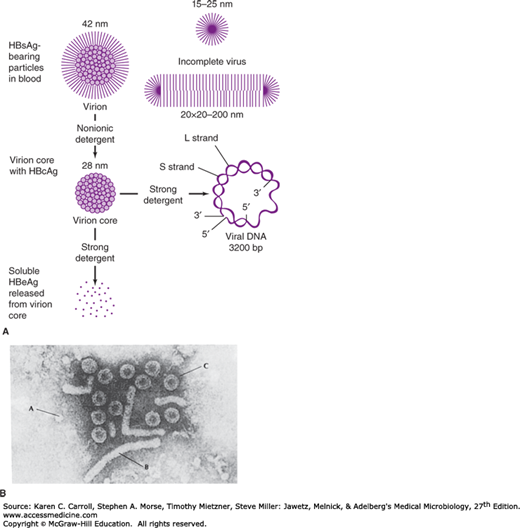
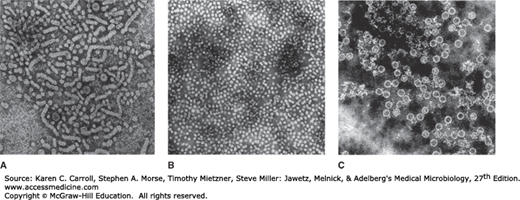
Electron microscopy of hepatitis B surface antigen (HBsAg)-positive serum reveals three morphologic forms (Figures 35-2 and 35-3A). The most numerous are spherical particles measuring 22 nm in diameter (Figure 35-3B). These small particles are made up exclusively of HBsAg—as are tubular or filamentous forms, which have the same diameter but may be more than 200 nm long—and result from overproduction of HBsAg. Larger, 42-nm spherical virions (originally referred to as Dane particles) are less frequently observed (see Figure 35-2). The outer surface, or envelope, contains HBsAg and surrounds a 27-nm inner nucleocapsid core that contains hepatitis B core antigen (HBcAg) (Figure 35-3C). The variable length of a single-stranded region of the circular DNA genome results in genetically heterogeneous particles with a wide range of buoyant densities.
FIGURE 35-2
Hepatitis B viral and subviral forms. A: Schematic representation of three hepatitis B surface antigen (HBsAg)-containing forms that can be identified in serum from hepatitis B virus (HBV) carriers. The 42-nm spherical Dane particle can be disrupted by nonionic detergents to release the 28-nm core that contains the partially double-stranded viral DNA genome. A soluble antigen, termed hepatitis B e antigen (HBeAg), may be released from core particles by treatment with strong detergent. HBcAg, hepatitis B core antigen. B: Electron micrograph showing three distinct HBsAg-bearing forms: 20-nm pleomorphic spherical particles (A), filamentous forms (B), and 42-nm spherical Dane particles, the infectious form of HBV (C). (Courtesy of FB Hollinger.)
FIGURE 35-3
A: Unfractionated hepatitis B surface antigen (HBsAg)-positive human plasma. Filaments, 22-nm spherical particles, and a few 42-nm virions are shown (77,000 ×). B: Purified HBsAg (55,000 ×). (Courtesy of RM McCombs and JP Brunschwig.) C: Hepatitis B core antigen purified from infected liver nuclei (122,400 ×). The diameter of the core particles is 27 nm. (Courtesy of HA Fields, GR Dreesman, and G Cabral.)
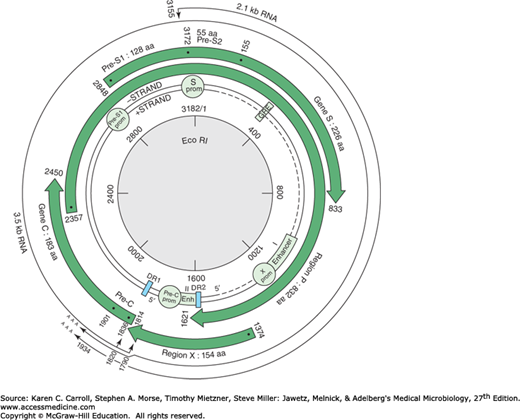
The viral genome (Figure 35-4) consists of partially double-stranded circular DNA, 3200 bp in length. Different HBV isolates share 90–98% nucleotide sequence homology. The full-length DNA minus strand (L or long strand) is complementary to all HBV mRNAs; the positive strand (S or short strand) is variable and between 50% and 80% of unit length.
FIGURE 35-4
Genetic organization of the hepatitis B virus genome. Four open reading frames encoding seven peptides are indicated by large arrows. Regulatory sequences (promoters [prom], enhancers [Enh], and glucocorticoid-responsive element [GRE]) are marked. Only the two major transcripts (core/pre-genome and S mRNAs) are represented. DR1 and DR2 are two directly repeated sequences of 11 bp at the 5′ extremities of the minus- and plus-strand DNA. (Reproduced with permission from Buendia MA: Hepatitis B viruses and hepatocellular carcinoma. Adv Cancer Res 1992;59:167. Academic Press, Inc.)
There are four open reading frames that encode seven polypeptides. These include structural proteins of the virion surface and core, a small transcriptional transactivator (X), and a large polymerase (P) protein that includes DNA polymerase, reverse transcriptase, and RNase H activities. The S gene has three in-frame initiation codons and encodes the major HBsAg, as well as polypeptides containing in addition pre-S2 or pre-S1 and pre-S2 sequences. The C gene has two in-frame initiation codons and encodes HBcAg plus the HBe protein, which is processed to produce soluble hepatitis B e antigen (HBeAg).
The particles containing HBsAg are antigenically complex. Each contains a group-specific antigen, a, in addition to two pairs of mutually exclusive subdeterminants, d/y and w/r. Thus, four phenotypes of HBsAg have been observed: adw, ayw, adr, and ayr. In the United States, adw is the predominant subtype. These virus-specific markers are useful in epidemiologic investigations because secondary cases have the same subtype as the index case.
The stability of HBsAg does not always coincide with that of the infectious agent. However, both are stable at −20°C for more than 20 years and stable to repeated freezing and thawing. The virus also is stable at 37°C for 60 minutes and remains viable after being dried and stored at 25°C for at least 1 week. HBV (but not HBsAg) is sensitive to higher temperatures (100°C for 1 minute) or to longer incubation periods (60°C for 10 hours). HBsAg is stable at a pH of 2.4 for up to 6 hours, but HBV infectivity is lost. Sodium hypochlorite, 0.5% (eg, 1:10 chlorine bleach), destroys antigenicity within 3 minutes at low protein concentrations, but undiluted serum specimens require higher concentrations (5%). HBsAg is not destroyed by ultraviolet irradiation of plasma or other blood products, and viral infectivity may also resist such treatment.
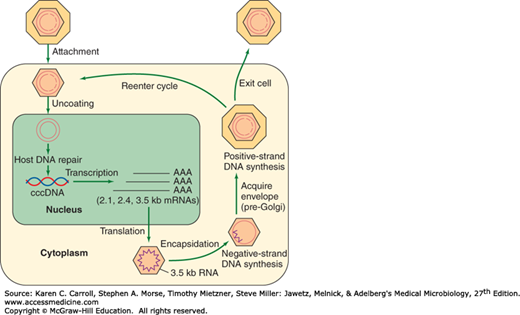
The infectious virion attaches to cells and becomes uncoated (Figure 35-5). In the nucleus, the partially double-stranded viral genome is converted to covalently closed circular double-stranded DNA (cccDNA). The cccDNA serves as template for all viral transcripts, including a 3.5-kb pregenome RNA. The pregenome RNA becomes encapsidated with newly synthesized HBcAg. Within the cores, the viral polymerase synthesizes by reverse transcription a negative-strand DNA copy. The polymerase starts to synthesize the positive DNA strand, but the process is not completed. Cores bud from the pre-Golgi membranes, acquiring HBsAg-containing envelopes, and may exit the cell. Alternatively, cores may be reimported into the nucleus and initiate another round of replication in the same cell.
FIGURE 35-5
Hepatitis B virus (HBV) replication cycle. HBV attachment to a receptor on the surface of hepatocytes occurs via a portion of the pre-S region of hepatitis B surface antigen (HBsAg). After uncoating of the virus, unidentified cellular enzymes convert the partially double-stranded DNA to covalent closed circular (ccc) DNA that can be detected in the nucleus. The cccDNA serves as the template for the production of HBV mRNAs and the 3.5-kb RNA pre-genome. The pre-genome is encapsidated by a packaging signal located near the 5′ end of the RNA into newly synthesized core particles, where it serves as template for the HBV reverse transcriptase encoded within the polymerase gene. An RNase H activity of the polymerase removes the RNA template as the negative-strand DNA is being synthesized. Positive-strand DNA synthesis does not proceed to completion within the core, resulting in replicative intermediates consisting of full-length minus-strand DNA plus variable-length (20–80%) positive-strand DNA. Core particles containing these DNA replicative intermediates bud from pre-Golgi membranes (acquiring HBsAg in the process) and may either exit the cell or reenter the intracellular infection cycle. (Reproduced with permission from Butel JS, Lee TH, Slagle BL: Is the DNA repair system involved in hepatitis-B-virus-mediated hepatocellular carcinogenesis? Trends Microbiol 1996;4:119.)
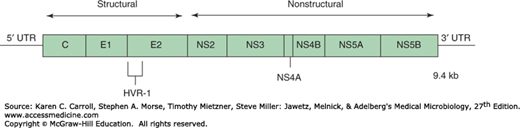
Clinical and epidemiologic studies and cross-challenge experiments in chimpanzees in the past had suggested that there were several non-A, non-B (NANB) hepatitis agents that, based on serologic tests, were not related to HAV or HBV. The major agent was identified as HCV. HCV is a positive-stranded RNA virus, classified as family Flaviviridae, genus Hepacivirus. Various viruses can be differentiated by RNA sequence analysis into at least six major genotypes (clades) and more than 100 subtypes. Clades differ from each other by 25–35% at the nucleotide level; subtypes differ from each other by 15–25%. The genome is 9.4 kb in size and encodes a core protein, two envelope glycoproteins, and several nonstructural proteins (Figure 35-6). The expression of cDNA clones of HCV in yeast led to the development of serologic tests for antibodies to HCV. Most cases of posttransfusion NANB hepatitis were caused by HCV.
FIGURE 35-6
Genetic organization of the hepatitis C virus genome. The single open reading frame is expressed as a polyprotein that gets processed; the positions of structural and nonstructural domains are shown. HVR-1 represents the highly variable region of an envelope glycoprotein. (Redrawn with permission from Chung RT, Liang TJ: Hepatitis C virus and hepatocellular carcinoma. In Parsonnet J [editor]. Microbes and Malignancy: Infection as a Cause of Human Cancers. Oxford University Press, 1999.)
Most new infections with HCV are subclinical. The majority (70–90%) of HCV patients develops chronic hepatitis, and many are at risk of progressing to chronic active hepatitis and cirrhosis (10–20%). In 1–5% of infected individuals, HCV leads to hepatocellular carcinoma, which is the fifth most common cause of cancer worldwide. About 25,000 individuals die annually of chronic liver disease and cirrhosis in the United States; HCV appears to be a major contributor to this burden (~40%).
The virus undergoes sequence variation during chronic infections. This complex viral population in a host is referred to as “quasi-species.” This genetic diversity is not correlated with differences in clinical disease, although differences do exist in response to antiviral therapy according to viral genotype.
An antigen–antibody system termed the delta antigen (delta-Ag) and antibody (anti-delta) is detected in some HBV infections. The antigen is found within certain HBsAg particles. In blood, HDV (delta agent) contains delta-Ag (HDAg) surrounded by an HBsAg envelope. It has a particle size of 35–37 nm and a buoyant density of 1.24–1.25 g/mL in CsCl. The genome of HDV consists of single-stranded, circular, negative-sense RNA, 1.7 kb in size. It is the smallest of known human pathogens and resembles subviral plant pathogens (ie, viroids). No homology exists with the HBV genome. HDAg is the only protein coded for by HDV RNA and is distinct from the antigenic determinants of HBV. HDV is a defective virus that requires the HBsAg coat for transmission. It is often associated with the most severe forms of hepatitis in HBsAg-positive patients. It is classified in the Deltavirus genus, which is not assigned to any virus family.
HEV is transmitted enterically and occurs in epidemic form in developing countries, where water or food supplies are sometimes fecally contaminated. It was first documented in samples collected during the New Delhi outbreak of 1955, when 29,000 cases of icteric hepatitis occurred after sewage contamination of the city’s drinking water supply. An epidemic occurred in Kashmir, India, in 1978, that resulted in an estimated 1700 deaths. Pregnant women may have a high (20%) mortality rate if fulminant hepatitis develops. The viral genome has been cloned and is a positive-sense, single-stranded RNA 7.2 kb in size. The virus is classified in the virus family, Hepeviridae, in the genus Hepevirus. HEV resembles, but is distinct from, caliciviruses. Animal strains of HEV are common throughout the world. There is evidence of HEV or HEV-like infections in rodents, pigs, sheep, and cattle in the United States, with occasional transmission to humans.
HEPATITIS VIRUS INFECTIONS IN HUMANS
Hepatitis is a general term meaning inflammation of the liver. Microscopically, there is spotty parenchymal cell degeneration, with necrosis of hepatocytes, a diffuse lobular inflammatory reaction, and disruption of liver cell cords. These parenchymal changes are accompanied by reticuloendothelial (Kupffer) cell hyperplasia, periportal infiltration by mononuclear cells, and cell degeneration. Localized areas of necrosis are frequently observed. Later in the course of the disease, there is an accumulation of macrophages near degenerating hepatocytes. Preservation of the reticulum framework allows hepatocyte regeneration so that the highly ordered architecture of the liver lobule can be ultimately regained. The damaged hepatic tissue is usually restored in 8–12 weeks.
Chronic carriers of HBsAg may or may not have demonstrable evidence of liver disease. Persistent (unresolved) viral hepatitis, a mild benign disease that may follow acute hepatitis B in 8–10% of adult patients, is characterized by sporadically abnormal aminotransferase values and hepatomegaly. Histologically, the lobular architecture is preserved, with portal inflammation, swollen and pale hepatocytes (cobblestone arrangement), and slight to absent fibrosis. This lesion is frequently observed in asymptomatic carriers, usually does not progress toward cirrhosis, and has a favorable prognosis.
Chronic active hepatitis features a spectrum of histologic changes from inflammation and necrosis to collapse of the normal reticulum framework with bridging between the portal triads or terminal hepatic veins. HBV is detected in 10–50% of these patients.
Occasionally during acute viral hepatitis, more extensive damage may occur that prevents orderly liver cell regeneration. Such fulminant or massive hepatocellular necrosis is seen in 1–2% of jaundiced patients with hepatitis B. It is 10 times more common in those coinfected with HDV than in the absence of HDV.
Both HBV and HCV have significant roles in the development of hepatocellular carcinoma that may appear many (15–60) years after establishment of chronic infection.
The clinical features of infections by HAV, HBV, and HCV are summarized in Table 35-4. In individual cases, it is not possible to make a reliable clinical distinction among cases caused by the hepatitis viruses.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree