51
Hemostasis & Thrombosis
Peter L. Gross, MD, MSc, FRCP(C), Robert K. Murray, MD, PhD, & Margaret L. Rand, PhD
OBJECTIVES
After studying this chapter, you should be able to:
![]() Understand the significance of hemostasis and thrombosis in health and disease.
Understand the significance of hemostasis and thrombosis in health and disease.
![]() Outline the pathways of coagulation that result in the formation of fibrin.
Outline the pathways of coagulation that result in the formation of fibrin.
![]() Identify the vitamin K-dependent coagulation factors.
Identify the vitamin K-dependent coagulation factors.
![]() Provide examples of genetic disorders that lead to bleeding.
Provide examples of genetic disorders that lead to bleeding.
![]() Describe the process of fibrinolysis.
Describe the process of fibrinolysis.
![]() Outline the steps leading to platelet aggregation.
Outline the steps leading to platelet aggregation.
![]() Identify the antiplatelet drugs and their mode of inhibition of platelet aggregation.
Identify the antiplatelet drugs and their mode of inhibition of platelet aggregation.
BIOMEDICAL IMPORTANCE
Basic aspects of the proteins of the blood coagulation system and of fibrinolysis are described in this chapter. Some fundamental aspects of platelet biology are also presented. Hemorrhagic and thrombotic states can cause serious medical emergencies, and thromboses in the coronary and cerebral arteries are major causes of death in many parts of the world. Rational management of these conditions requires a clear understanding of the bases of blood coagulation, fibrinolysis, and platelet activation.
HEMOSTASIS&THROMBOSIS HAVE THREE COMMON PHASES
Hemostasis is the cessation of bleeding from a cut or severed vessel, whereas thrombosis occurs when the endothelium lining blood vessels is damaged or removed (eg, upon rupture of an atherosclerotic plaque). These processes involve blood vessels, platelet aggregation, and plasma proteins that cause formation or dissolution of platelet aggregates and fibrin.
In hemostasis, there is initial vasoconstriction of the injured vessel, causing diminished blood flow distal to the injury. Then, hemostasis and thrombosis share three phases:
1. Formation of a loose and temporary platelet aggregate at the site of injury. Platelets bind to collagen at the site of vessel wall injury, and form thromboxane A2 and release ADP, which activate other platelets flowing by the vicinity of the injury. (The mechanism of platelet activation is described below.) Thrombin, formed during coagulation at the same site, causes further platelet activation. Upon activation, platelets change shape and, in the presence of fibrinogen and/or von Willebrand factor, aggregate to form the hemostatic plug (in hemostasis) or thrombus (in thrombosis).
2. Formation of a fibrin mesh that binds to the platelet aggregate, forming a more stable hemostatic plug or thrombus.
3. Partial or complete dissolution of the hemostatic plug or thrombus by plasmin.
There Are Three Types of Thrombi
Three types of thrombi or clots are distinguished. All three contain fibrin in various proportions.
1. The white thrombus is composed of platelets and fibrin and is relatively poor in erythrocytes. It forms at the site of an injury or abnormal vessel wall, particularly in areas where blood flow is rapid (arteries).
2. The red thrombus consists primarily of red cells and fibrin. It morphologically resembles the clot formed in a test tube and may form in vivo in areas of retarded blood flow or stasis (eg, veins) with or without vascular injury, or it may form at a site of injury or in an abnormal vessel in conjunction with an initiating platelet plug.
3. A third type is fibrin deposits in very small blood vessels or capillaries.
We shall first describe the coagulation pathway leading to the formation of fibrin. Then, we shall briefly describe some aspects of the involvement of platelets and blood vessel walls in the overall process. This separation of clotting factors and platelets is artificial since both play intimate and often mutually interdependent roles in hemostasis and thrombosis, but it facilitates description of the overall processes involved.
Both Extrinsic & Intrinsic Pathways Result in the Formation of Fibrin
Two pathways lead to fibrin clot formation: the extrinsic and the intrinsic pathways. These pathways are not independent, as previously thought. However, this artificial distinction is retained in the following text to facilitate their description.
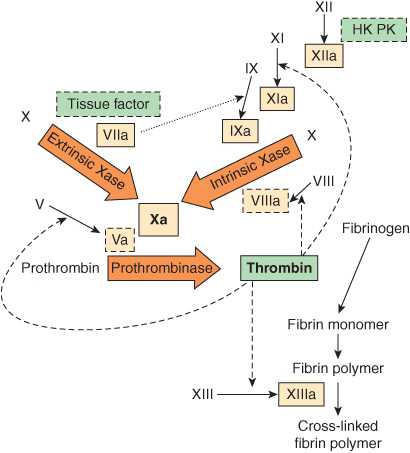
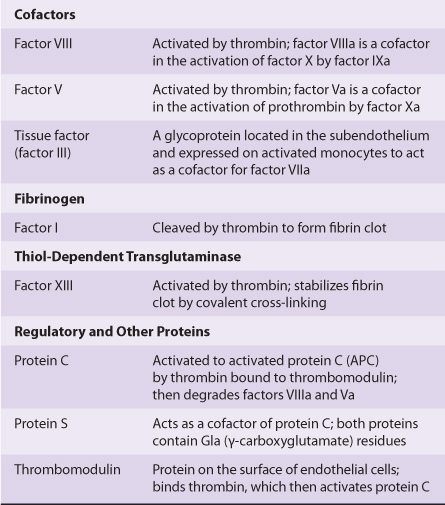
Initiation of fibrin clot formation in response to tissue injury is carried out by the extrinsic pathway. The intrinsic pathway is activated by negatively charged surfaces in vitro, eg, glass. Both pathways lead to activation of prothrombin to thrombin and the thrombin-catalyzed cleavage of fibrinogen to form the fibrin clot. The pathways are complex and involve many different proteins (Figures 51-1 and 51-2; Table 51-1). In general, as shown in Table 51-2, these proteins can be classified into five types: (1) zymogens of serine-dependent proteases, which become activated during the process of coagulation; (2) cofactors; (3) fibrinogen; (4) a transglutaminase, which stabilizes the fibrin clot; and (5) regulatory and other proteins.
FIGURE 51–1 The pathways of blood coagulation, with the extrinsic pathway indicated at the top left and the intrinsic pathway at the top right. The pathways converge in the formation of factor Xa and culminate in the formation of cross-linked fibrin. Complexes of tissue factor and factor VIIa activate not only factor X (extrinsic Xase [tenase]) but also factor IX in the intrinsic pathway (dotted arrow). In addition, thrombin feedback activates at the sites indicated (dashed arrows) and also activates factor VII to factor VIIa (not shown). The three predominant complexes, extrinsic Xase, intrinsic Xase, and prothrombinase, are indicated in the arrows; the reactions require anionic procoagulant phospholipid membrane and calcium. Activated proteases are in solid-outlined boxes; active cofactors are in dash-outlined boxes and inactive factors are not in boxes. (HK, high-molecular-weight kininogen; PK, prekallikrein.)
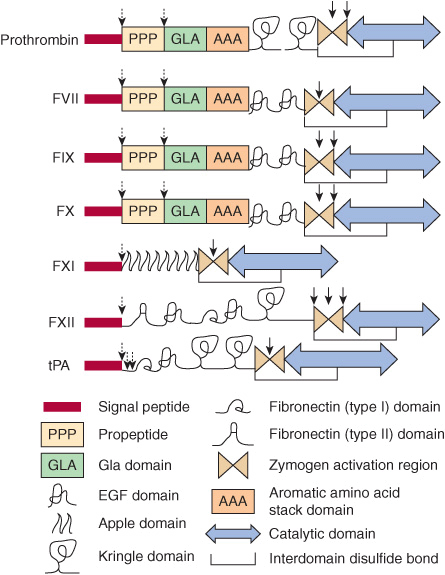
FIGURE 51–2 The structural domains of selected proteins involved in coagulation and fibrinolysis. The domains are as identified at the bottom of the figure and include signal peptide, propeptide, Gla (γ-carboxyglutamate) domain, epidermal growth factor (EGF) domain, apple domain, kringle domain, fibronectin (types I and II) domain, the zymogen activation region, aromatic amino acid stack, and the catalytic domain. Interdomain disulfide bonds are indicated, but numerous intradomain disulfide bonds are not. Sites of proteolytic cleavage in synthesis or activation are indicated by arrows (dashed and solid, respectively). FVII, factor VII; FIX, factor IX; FX, factor X, FXI; factor XI; FXII, factor XII; tPA, tissue plasminogen activator. (Adapted, with permission, from Furie B, Furie BC: The molecular basis of blood coagulation. Cell 1988;53:505.)
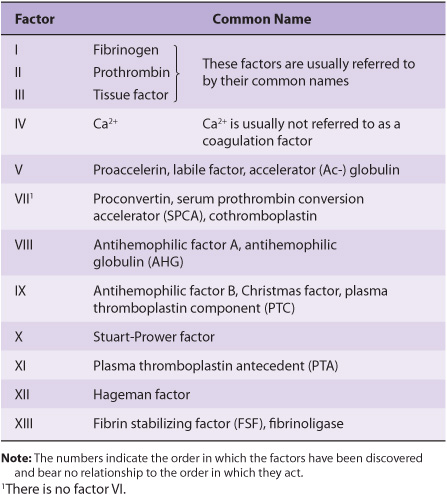
TABLE 51–1 Numerical System for Nomenclature of Blood Clotting Factors
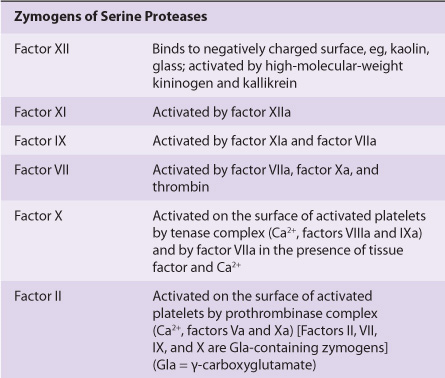
TABLE 51–2 The Functions of the Proteins Involved in Blood Coagulation
The Extrinsic Pathway Leads to Activation of Factor X
The extrinsic pathway involves tissue factor, factors VII and X, and Ca2+ and results in the production of factor Xa (by convention, activated clotting factors are referred to by use of the suffix a). It is initiated at the site of tissue injury with the exposure of tissue factor (Figure 51–1), located in the subendothelium and on activated monocytes. Tissue factor interacts with and activates factor VII (53 kDa, a zymogen containing vitamin K-dependent γ -carboxyglutamate [Gla] residues; see Chapter 44), synthesized in the liver. It should be noted that in the Gla-containing zymogens (factors II, VII, IX, and X), the Gla residues in the amino terminal regions of the molecules serve as high-affinity binding sites for Ca2+. Tissue factor acts as a cofactor for factor VIIa, enhancing its enzymatic activity to activate factor X (56 kDa). The reaction by which factor X is activated requires the assembly of components, termed the extrinsic tenase complex, on a cell membrane surface exposing the procoagulant phospholipid phosphatidylserine; these components are Ca2+, tissue factor, factor VIIa, and factor X. Factor VIIa cleaves an Arg-Ile bond in factor X to produce the two-chain serine protease, factor Xa. Tissue factor and factor VIIa also activate factor IX in the intrinsic pathway. Indeed, the formation of complexes between tissue factor and factor VIIa is now considered to be the key process involved in initiation of blood coagulation in vivo.
Tissue factor pathway inhibitor (TFPI) is a major physiologic inhibitor of coagulation. It is a protein that circulates in the blood associated with lipoproteins. TFPI directly inhibits factor Xa by binding to the enzyme near its active site. This factor Xa-TFPI complex then inhibits the factor VIIa-tissue factor complex.
The Intrinsic Pathway Also Leads to Activation of Factor X
The activation of factor Xa is the major site where the intrinsic and extrinsic pathways converge (Figure 51–1). The intrinsic pathway (Figure 51–1) involves factors XII, XI, IX, VIII, and X as well as prekallikrein, high-molecular-weight (HMW) kininogen, Ca2+, and phospholipid. It results in the production of factor Xa that is cleaved by the tenase complex, with factor IXa as the serine protease and factor VIIIa as the cofactor, of the intrinsic pathway. Activation of factor X provides an important link between the intrinsic and extrinsic pathways.
The intrinsic pathway can be initiated with the “contact phase” in which prekallikrein, HMW kininogen, factor XII, and factor XI are exposed to a negatively charged activating surface. Kaolin can be used for in vitro tests as an initiator of the intrinsic pathway. When the components of the contact phase assemble on the activating surface, factor XII is activated to factor XIIa upon proteolysis by kallikrein. This factor XIIa, generated by kallikrein, attacks prekallikrein to generate more kallikrein, setting up a reciprocal activation. Factor XIIa, once formed, activates factor XI to XIa and also releases bra-dykinin (a nonapeptide with potent vasodilator action) from HMW kininogen.
Factor XIa in the presence of Ca2+ activates factor IX (55 kDa, a Gla-containing zymogen), to the serine protease, factor IXa. This, in turn, also cleaves an Arg-Ile bond in factor X to produce factor Xa. This latter reaction requires the assembly of components, called the intrinsic tenase complex, on a membrane surface: Ca2+ and factor VIIIa, as well as factors IXa and X.
Factor VIII (330 kDa), a circulating glycoprotein, is not a protease precursor but a cofactor that serves as a receptor for factors IXa and X on the platelet surface. Factor VIII is activated by minute quantities of thrombin to form factor VIIIa, which is in turn inactivated upon further cleavage by thrombin.
The role of the initial steps of the intrinsic pathway in initiating coagulation has been called into question because patients with a hereditary deficiency of factor XII, prekallikrein or HMW kininogen do not exhibit bleeding problems. Similarly, patients with a deficiency of factor XI may not have bleeding problems. The intrinsic pathway largely serves to amplify factor Xa and ultimately thrombin formation, through feedback mechanisms (see below). The intrinsic pathway may also be important in fibrinolysis (see below) since kallikrein, factor XIIa, and factor XIa can cleave plasminogen and kallikrein can activate single-chain urokinase.
Factor Xa Leads to Activation of Prothrombin to Thrombin
Factor Xa, produced by either the extrinsic or the intrinsic pathway, activates prothrombin (factor II) to thrombin (factor IIa) (Figure 51–1).
The activation of prothrombin, like that of factor X, occurs on a membrane surface and requires the assembly of a prothrombinase complex, consisting of Ca2+, factor Va, factor Xa, and prothrombin. The assembly of the prothrombinase and tenase complexes takes place on the membrane surface of platelets activated to expose the acidic (anionic) phospholipid phosphatidylserine, which is normally on the internal side of the plasma membrane of resting, nonactivated platelets.
Factor V (330 kDa), a glycoprotein with homology to factor VIII and ceruloplasmin, is synthesized in the liver, spleen, and kidney and is found in platelets as well as in plasma. It functions as a cofactor in a manner similar to that of factor VIII in the tenase complex. When activated to factor Va by traces of thrombin, it binds specifically to the platelet membrane (Figure 51–3) and forms a complex with factor Xa and prothrombin. It is subsequently inactivated by activated protein C (see below), thereby providing a means of limiting the activation of prothrombin to thrombin. Prothrombin (72 kDa; Figure 51–3) is a single-chain glycoprotein synthesized in the liver. The amino terminal region of prothrombin (Figure 51–2) contains 10 Gla residues, and the serine-dependent active protease site is in the catalytic domain close to the carboxyl terminal region of the molecule. Upon binding to the complex of factors Va and Xa on the platelet membrane (Figure 51–3), prothrombin is cleaved by factor Xa at two sites to generate the active, two-chain thrombin molecule, which is then released from the platelet surface.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree