STEM CELLS, GROWTH FACTORS, & DIFFERENTIATION
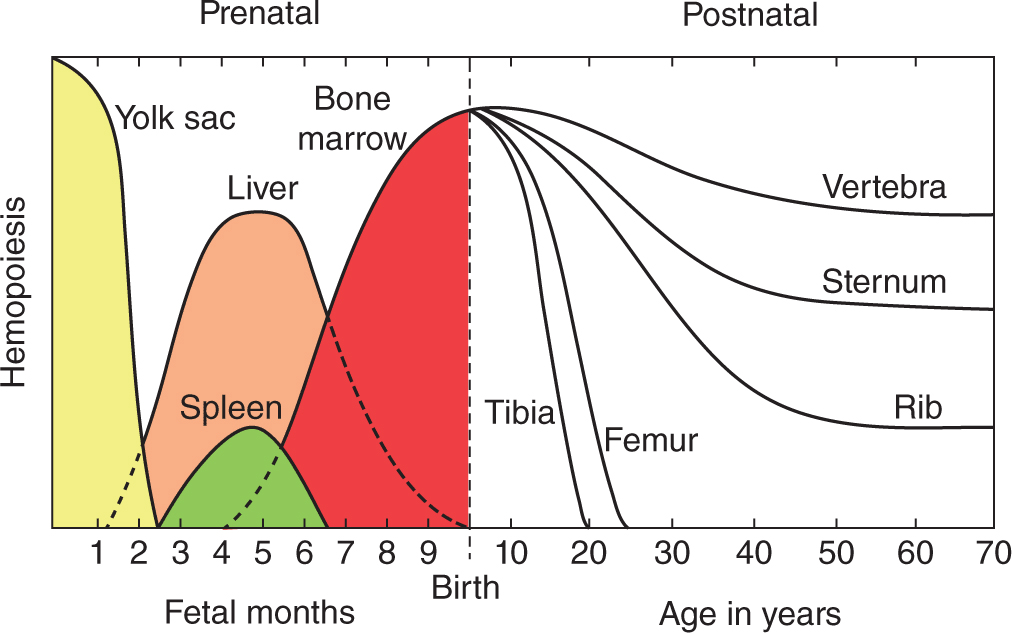
Mature blood cells have a relatively short life span and must be continuously replaced with new cells from precursors developing during hemopoiesis (Gr. haima, blood + poiesis, a making). In the early embryo these blood cells arise in the yolk sac mesoderm. In the second trimester, hemopoiesis (also called hematopoiesis) occurs primarily in the developing liver, with the spleen playing a more minor role (Figure 13–1). Skeletal elements begin to ossify and bone marrow develops in their medullary cavities, so that in the third trimester marrow of specific bones becomes the major hemopoietic organ.
FIGURE 13–1 Shifting locations of hemopoiesis during development and aging.
Throughout childhood and adult life, erythrocytes, granulocytes, monocytes, and platelets continue to form from stem cells located in bone marrow. The origin and maturation of these cells are termed, respectively, erythropoiesis (Gr. erythros, red + poiesis), granulopoiesis, monocytopoiesis, and thrombocytopoiesis. As described in Chapter 14 on the immune system, lymphopoiesis or lymphocyte development occurs in the marrow and in the lymphoid organs to which precursor cells migrate from marrow.
This chapter describes the stem and progenitor cells of hemopoiesis and their controlling factors, the histology of bone marrow, the major stages of red and white blood cell differentiation, and platelet formation.
STEM CELLS, GROWTH FACTORS, & DIFFERENTIATION
As discussed in Chapter 3, stem cells are pluripotent cells capable of asymmetric division and self-renewal. Some of their daughter cells form specific, irreversibly differentiated cell types, and other daughter cells remain as a small pool of slowly dividing stem cells.
Hemopoietic stem cells can be isolated by using fluorescence-labeled antibodies to mark specific cell surface antigens and passing the cell population through a fluorescence-activated cell-sorting (FACS) instrument. Stem cells are studied using experimental techniques that permit analysis of hemopoiesis in vivo and in vitro.
In vivo techniques include injecting the bone marrow of normal donor mice into irradiated mice whose hematopoietic cells have been destroyed. In these animals, only the transplanted bone marrow cells produce hematopoietic colonies in the bone marrow and spleen, simplifying studies of this process. This work led to the clinical use of bone marrow transplants to treat potentially lethal hemopoietic disorders.
In vitro techniques using semisolid tissue culture media containing substances produced by marrow stromal cells are used to identify and study the cytokines promoting hemopoietic cell growth and differentiation.
Hemopoietic Stem Cells
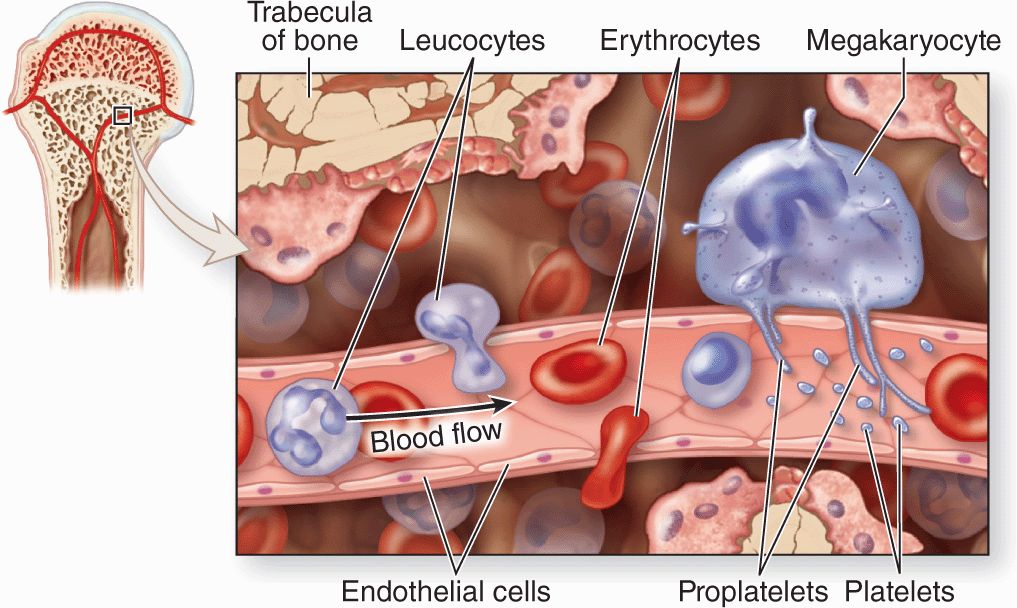
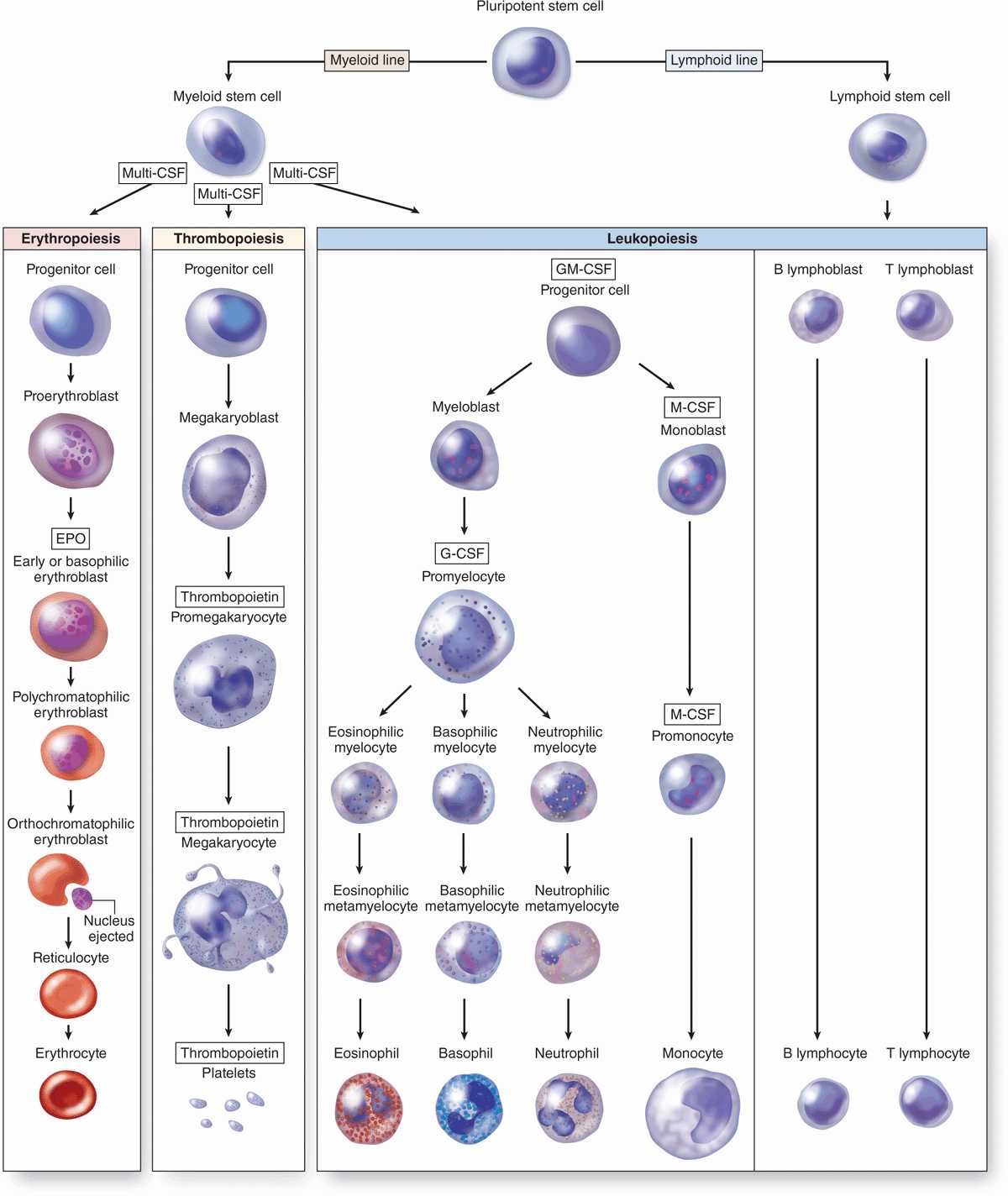
All blood cells arise from a single major type of pluripotent stem cell in the bone marrow that can give rise to all the blood cell types (Figure 13–2). These pluripotent stem cells are rare, but they proliferate and form two major lineages of progenitor cells with restricted potentials (committed to produce specific blood cells): one for lymphoid cells (lymphocytes) and another for myeloid cells (Gr. myelos, marrow) that develop in bone marrow. Myeloid cells include granulocytes, monocytes, erythrocytes, and megakaryocytes. As described in Chapter 14 on the immune system, the lymphoid progenitor cells migrate from the bone marrow to the thymus or the lymph nodes, spleen, and other lymphoid structures, where they proliferate and differentiate.
FIGURE 13–2 Origin and differentiative stages of blood cells.
Progenitor & Precursor Cells
The progenitor cells for blood cells are commonly called colony-forming units (CFUs), because they give rise to colonies of only one cell type when cultured or injected into a spleen. As shown in Figure 13–2, there are four major types of progenitor cells/CFUs:
 Erythroid lineage of CFU-erythrocytes (CFU-E),
Erythroid lineage of CFU-erythrocytes (CFU-E),
 Thrombocytic lineage of CFU-megakaryocytes (CFU-Meg),
Thrombocytic lineage of CFU-megakaryocytes (CFU-Meg),
 Granulocyte-monocyte lineage of CFU-granulocytes-monocytes (CFU-GM), and
Granulocyte-monocyte lineage of CFU-granulocytes-monocytes (CFU-GM), and
 Lymphoid lineage of CFU-lymphocytes of all types (CFU-L).
Lymphoid lineage of CFU-lymphocytes of all types (CFU-L).
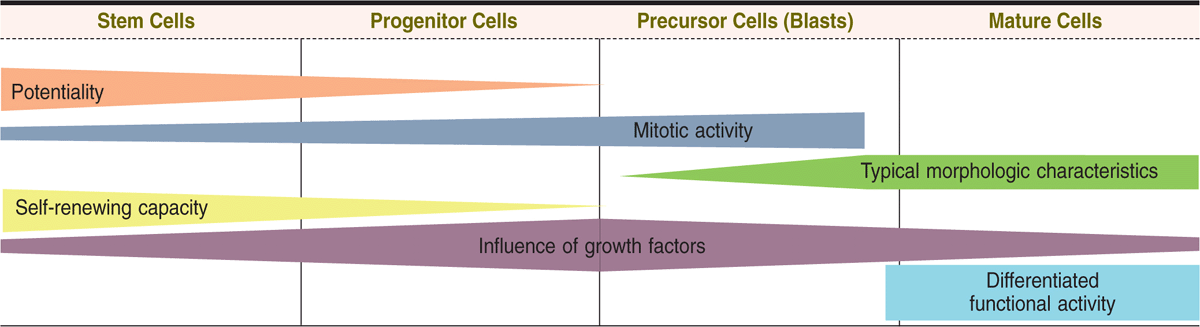
Each progenitor cell/CFU lineage produces precursor cells (or blasts) that gradually assume the morphologic characteristics of the mature, functional cell types they will become (Figure 13–2). In contrast, stem and progenitor cells cannot be morphologically distinguished and simply resemble large lymphocytes. While stem cells divide at a rate only sufficient to maintain their relatively small population, progenitor and precursor cells divide more rapidly, producing large numbers of differentiated, mature cells (3 × 109 erythrocytes and 0.85 × 109 granulocytes/kg/d in human bone marrow). The changing potential and activities of cells during hemopoiesis are shown graphically in Figure 13–3.
FIGURE 13–3 Major changes in developing hemopoietic cells.
Hemopoiesis depends on a microenvironment, or niche, with specific endocrine, paracrine, and juxtacrine factors. These requirements are provided largely by the local cells and extracellular matrix (ECM) of the hemopoietic organs, which together create the niches in which stem cells are maintained and progenitor cells develop.
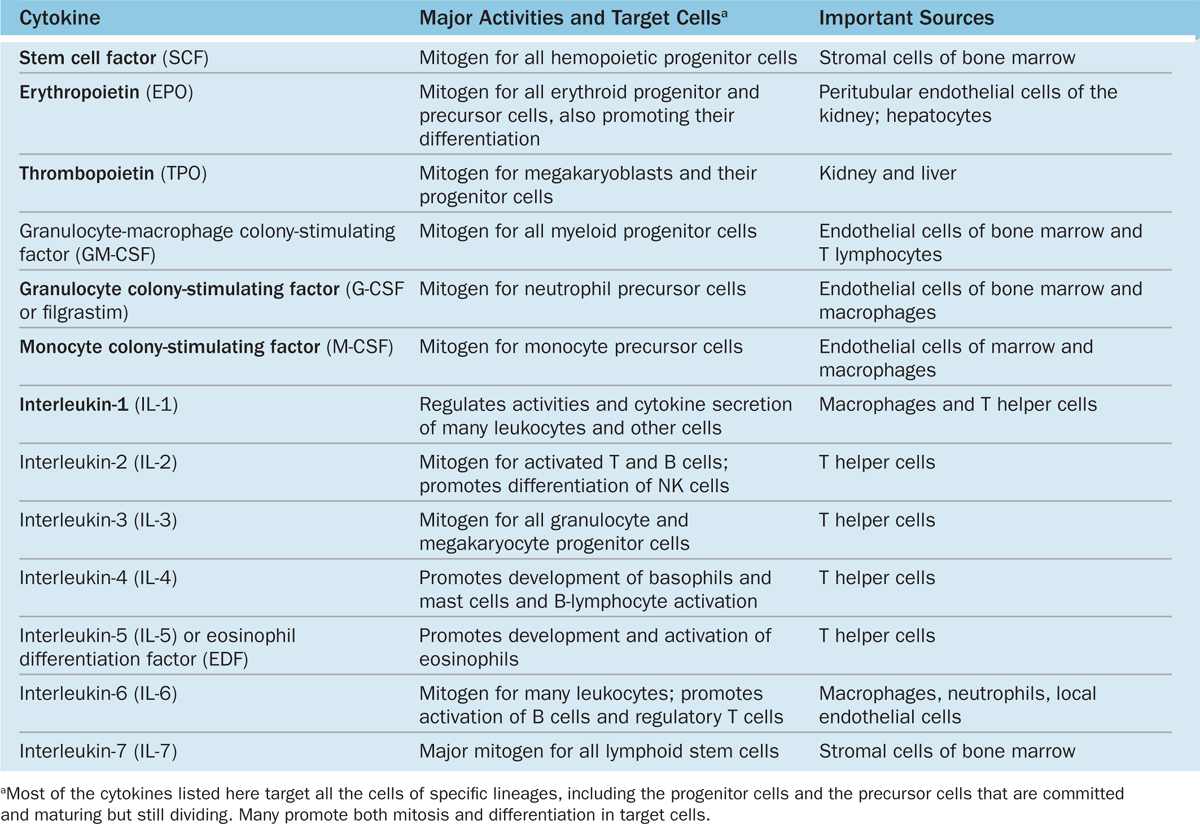
Hemopoietic growth factors, often called colony-stimulating factors (CSF) or cytokines, are glycoproteins that stimulate proliferation of progenitor and precursor cells and promote cell differentiation and maturation within specific lineages. Cloning of the genes for several important hematopoietic growth factors has significantly advanced study of blood formation and permitted the production of clinically useful factors for patients with hemopoietic disorders. The major activities, target cells, and sources of several well-characterized cytokines promoting hemopoiesis are presented in Table 13–1.
TABLE 13–1 Major hemopoietic cytokines (growth factors or colony-stimulating factors).
BONE MARROW
Under normal conditions, the production of blood cells by the bone marrow is adjusted to the body’s needs, increasing its activity several-fold in a very short time. Bone marrow is found in the medullary canals of long bones and in the small cavities of cancellous bone, with two types based on their appearance at gross examination: blood-forming red bone marrow, whose color is produced by an abundance of blood and hemopoietic cells, and yellow bone marrow, which is filled with adipocytes that exclude most hemopoietic cells. In the newborn all bone marrow is red and active in blood cell production, but as the child grows, most of the marrow changes gradually to the yellow variety. Under certain conditions, such as severe bleeding or hypoxia, yellow marrow reverts to red.
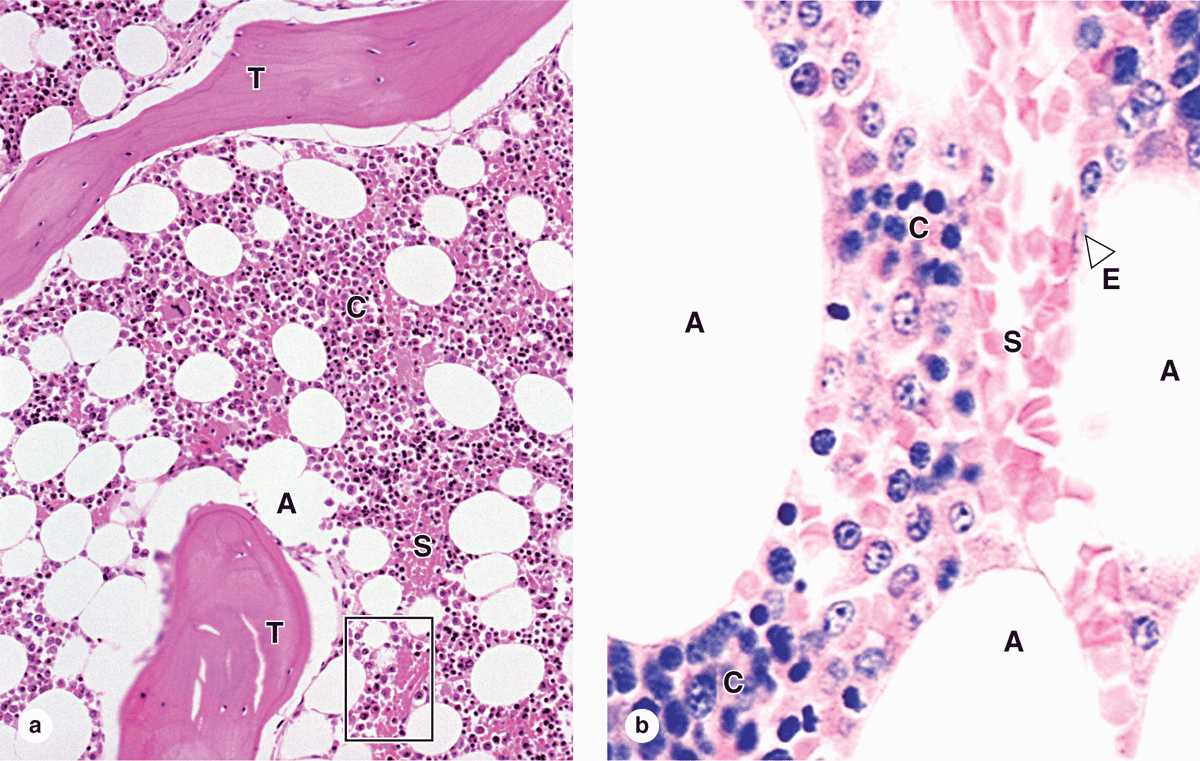
Red bone marrow (Figure 13–4) contains a reticular connective tissue stroma (Gr. stroma, bed), hemopoietic cords or islands of cells, and sinusoidal capillaries. The stroma is a meshwork of specialized fibroblastic cells called stromal cells (also called reticular or adventitial cells) and a delicate web of reticular fibers supporting the hemopoietic cells and macrophages. The matrix of bone marrow also contains collagen type I, proteoglycans, fibronectin, and laminin, the latter glycoproteins interacting with integrins to bind cells to the matrix. Red marrow is also a site where older, defective erythrocytes undergo phagocytosis by macrophages, which then reprocess heme-bound iron for delivery to the differentiating erythrocytes.
FIGURE 13–4 Red bone marrow (active in hemopoiesis).
The hematopoietic niche in marrow includes the stroma, osteoblasts, and megakeryocytes. Between the hematopoietic cords run the sinusoids, which have discontinuous endothelium, through which newly differentiated blood cells and platelets enter the circulation (Figure 13–5).
FIGURE 13–5 Sinusoidal endothelium in active marrow.
 MEDICAL APPLICATION
MEDICAL APPLICATION