Heart Failure
LEARNING OBJECTIVE
• INTRODUCTION
Heart failure (HF), also known as congestive or chronic heart failure, is a significant complication of essential hypertension and atherosclerotic cardiovascular disease. In 2010, more than 6 million people had a diagnosis of heart failure. Pharmacists in a primary care or disease management role routinely assist patients in managing their diabetes, hypertension, and dyslipidemia, all contributing factors to the development of heart failure as a complication of their chronic diseases. Therefore, it is important for pharmacists to understand the clinical course and pathophysiology and to be able to identify signs and symptoms of heart failure.
• ETIOLOGY
President Franklin D. Roosevelt suffered from severe hypertension. During World War II, he developed congestive heart failure as a complication of his uncontrolled hypertension and eventually died of a massive stroke. In his time, hypertension was almost the sole cause of congestive heart failure. However, as we developed drugs to treat hypertension and focused on controlling elevated blood pressure, it became apparent that heart failure had multiple causes. While more than three-fourths of all patients with HF have a history of hypertension, it is now known that heart failure is a complex syndrome where any structural or functional cardiac damage can impair the ability of the ventricles to fill with or eject blood. Heart failure is the result of continual overactivation of the sympathetic nervous system, the renin-angiotensin-aldosterone (RAA) system, as well as the release of other neurohormones, such as B-type natriuretic peptide (BNP), all of which contribute to the pathophysiology of heart failure. The terms preload and afterload are frequently used when discussing heart failure and other cardiovascular diseases. Preload is defined as the amount of blood presented to the heart for pumping. Preload is increased in heart failure. Diuretics, a mainstay of the treatment of heart failure, decrease blood volume presented to the heart for pumping and, therefore, decrease preload. Afterload is defined as the amount of peripheral resistance the heart pumps blood against, i.e. arterial hypertension. Afterload is generally increased in heart failure in the form of hypertension. Many antihypertensives decrease afterload through their effects on reducing peripheral resistance caused by the sympathetic and RAA systems. This combination of increased preload and afterload in heart failure causes a cyclical worsening of heart failure. Both the sympathetic and RAA systems cause remodeling of the ventricles, which eventually leads to left ventricular enlargement, and ventricular wall stiffness and reduced cardiac output.
Patients with heart failure are currently classified as having systolic or diastolic cardiac dysfunction based on left ventricular ejection fraction (LVEF). In both types of heart failure, cardiac output is markedly reduced, resulting in decreased perfusion of peripheral tissues and organs. In patients with heart failure with systolic dysfunction or heart failure with reduced ejection fraction (HFREF), the ejection fraction (EF) is <40% (>60% is normal). This represents a left ventricle that is enlarged and weakened to the extent that it cannot empty the ventricle completely. This most closely corresponds to classic congestive heart failure where uncontrolled hypertension causes the left ventricle to hypertrophy and eventually fail. In patients with diastolic dysfunction or heart failure with preserved ejection fraction (HFPEF), the ejection fraction is between 40% and 55%, which represents a stiff heart that has lost its flexibility and cannot completely fill by stretching, leading to a decreased cardiac output. The major cause of diastolic dysfunction in heart failure is coronary artery disease.
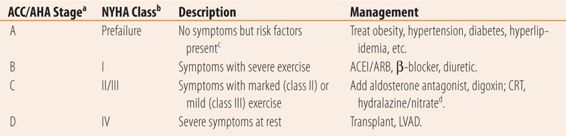
The severity of the heart failure can also be classified. ACC/AHA and New York Heart Association Classification Systems break down severity into four major groups based on activity and symptoms (Table 22.1).
| TABLE 22.1 | Classification and Treatment of Chronic Heart Failure |
• DIAGNOSIS
Subjective findings in heart failure are classical. In heart failure shortness of breath (SOB) is the attempt of the body to compensate for poor perfusion of peripheral tissue (hypoxia), by increasing the rate and depth of respiration. When it occurs during exertion, e.g., walking up stairs or an incline, it is called dyspnea on exertion (DOE). Paroxysmal nocturnal dyspnea (PND) is awakening 2 to 4 hours after lying down, with shortness of breath or coughing. Sitting up or standing up quickly relieves the symptom. During the day the excess fluid retained from heart failure pools in the lower extremities due to gravity and may manifest itself as ankle edema. When the patient lies down flat, this excess fluid redistributes itself into the intravascular space, markedly increasing preload. The failing heart decompensates and the excess fluid leaks into the lung tissue, causing the respiratory symptoms, a mechanism similar to acute pulmonary edema. By sitting or standing, the excess fluid repools in the lower extremities and the heart which now does not have to pump all the excess fluid is able work better and the fluid in the lungs eventually returns to the intravascular space, reliving the respiratory symptoms. Over time and with repeated episodes, patients begin to realize that they sleep better propped up with several pillows or in a recliner. This sleeping propped up is called orthopnea and is quantified by the number of pillows they use, e.g., two- or three-pillow orthopnea. Orthopnea is relieved in patients admitted for heart failure who are initially placed in a semi-Fowler position by elevating the head of hospital bed 30° to reduce the preload and allow them more restful sleep.
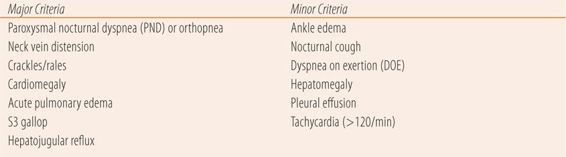
There are multiple objective findings in patients with heart failure. The more severe the heart failure, the more physical signs are seen. Crackles or rales on auscultation can be heard in both lung bases. Lower extremity edema in the ankles and feet (pedal edema) is common as are tachycardia and tachypnea as the body tries to compensate for peripheral tissue hypoxia. Many times the point of maximal impulse (PMI) or apical impulse will be displaced laterally and inferiorly indicating an enlarged heart. Other signs only occur in more severe disease such as internal jugular vein distention (JVD), the hepatojugular reflux (HJR), and an S3 gallop rhythm on cardiac auscultation. Chest x-ray and echocardiogram will show an enlarged heart and fluid in the lungs. An electrocardiogram may show left ventricular hypertrophy and left axis deviation. Renal function tests (serum creatinine and blood urea nitrogen) may be elevated due to poor kidney perfusion. Likewise venous congestion in the liver can cause elevated serum AST and ALT levels. These lab values return to normal after heart failure is treated and kidney perfusion improves and excessive preload is reduced. There are clinical diagnostic criteria for heart failure. The best known are the Framingham criteria seen in Table 22.2. Definite diagnosis of heart failure requires the presence of two major criteria or one major criterion plus two minor criteria provided other causes have been ruled out. One major new diagnostic test has been added to the array of tests used to confirm heart failure. B-type natriuretic peptide (brain natriuretic peptide or BNP) is released as a response to the degrees of ventricular stretch and ventricular dilation to help the heart compensate by its action to excrete fluid, sodium, and potassium. At levels >100 pg/mL, BNP has a sensitivity of 90% and a specificity of 73%. Levels above 100 pg/mL are diagnostic of heart failure in nonobese patients. BNP levels are particularly useful to rule out heart failure as a cause of dyspnea or to confirm heart failure in patients with marginal criteria for a clinical diagnosis. BNP also has prognostic value. Each 100 pg/mL increase in BNP is associated with a 35% increase in the relative risk of death. Also, in patients discharged from the hospital, a 46% reduction from admissions levels along with a value <300 pg/mL provided significant reductions in morbidity and mortality. NT pro-BNP, the precursor to BNP can also be used but it has different target and diagnostic values.
| TABLE 22.2 | Framingham Clinical Diagnostic Criteria for Congestive Heart Failure |
• MONITORING AT FOLLOW-UP VISITS
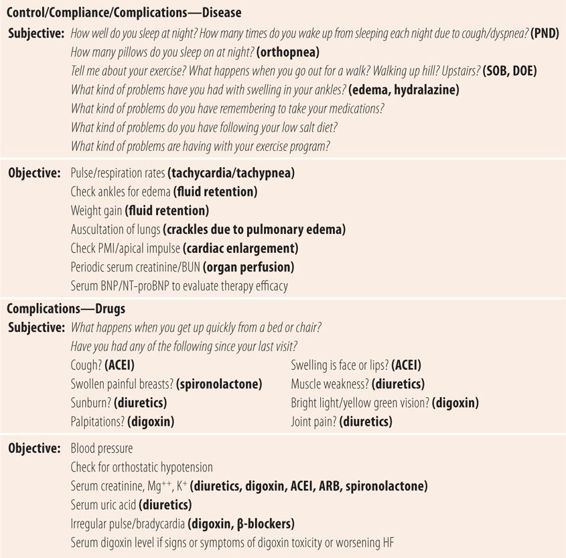
Treatment of heart failure is directed at reducing both preload and afterload, primarily through interfering with neurohormonal manifestations. Diuretics, usually loop diuretics, are the initial treatment of choice to remove excess fluid. Next, either an angiotensin-converting-enzyme inhibitor (ACEI) or an angiotensin-receptor blocker (ARB) decreases afterload and ameliorates ventricular remodeling caused by angiotensin. Higher rather than lower doses are preferred. β-Blockers reduce the negative effects of excessive sympathetic activity. The dose of β-blockers must be carefully titrated upward, beginning with low doses to prevent blocking of necessary sympathetic activity and worsening the heart failure. Aldosterone antagonists, such as spironolactone, improve survival and prevent myocardial fibrosis associated with heart failure. These four medications all improve both the morbidity and mortality in systolic dysfunction, but are significantly less effective in diastolic dysfunction where diuretics are the primary therapy. Digoxin, once the mainstay of HF treatment, is now relegated to use as a 5th agent in patients who fail to respond to the 4-drug regimen. The main limitation of this four-drug combination is hypotension, so at each visit careful history for orthostasis and checking for orthostatic hypotension are needed (see Chapter 18). In recent years, the prevalence of diastolic dysfunction has been increasing. Since many patients with diastolic dysfunction may also have some masked systolic dysfunction, new effective strategies are being employed using BNP levels to determine the level of aggressiveness of therapy in systolic dysfunction, to maximize the benefits of nondiuretic medications have benefit in mixed dysfunction heart failure, and to reduce the incidence of readmissions. The goal of BNP-targeted therapy is to reduce the level to as close to 100 pg/mL as possible without toxicity. See Table 22.3 for a more complete listing of parameters of control, compliance, and complications.
| TABLE 22.3 | Follow-Up Visit for Heart Failure |
• KEY REFERENCES
1. King M, Kingery J, Casey B. Diagnosis and evaluation of heart failure. Am Fam Physician. 2012;85:1161-1168.
2. Chatterjee K. Pathophysiology of systolic and diastolic heart failure. Med Clin North Am. 2012;96:891-899.
3. Maestre A, Gil V, Gallego J, et al. Diagnostic accuracy of clinical criteria for identifying systolic and diastolic heart failure: cross-sectional study. J Eval Clin Pract. 2009;15:55-61.
4. Motiwala SR, Januzzi JL. The role of natriuretic peptides for guiding management of chronic heart failure. Clin Pharmacol Ther. 2013;93:57-67.
5. Rathi S, Deedwania PC. The epidemiology and pathophysiology of heart failure. Med Clin North Am. 2012;96:881-890.
6. Campbell RT, Jhund PS, Castagno D, et al. What we have learned about patients with heart failure and preserved ejection fraction from DIG-PEF, CHARM-Preserved and I-Preserve. J Am Coll Cardiol. 2012;60:2349-2356.
7. Chowdhury P, Choudhary R, Maisel A. The appropriate use of biomarkers in heart failure. Med Clin North Am. 2012;96:901-913.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



