Asthma and Chronic Obstructive Pulmonary Disease (COPD)
LEARNING OBJECTIVES
• INTRODUCTION
Roughly 12% of the population of the United States suffers from either asthma or COPD, at an economic cost of over $100 billion annually. COPD is the third leading cause of death in the United States. As with other chronic diseases, pharmacists can play an important role in assisting patients to optimize the benefits from their medication regimen. The pharmacist, either as part of a health care delivery team or in pharmacist-run asthma or COPD disease management programs, has been shown to have a positive clinical and economic benefit on patient outcomes. Since medication is the primary therapeutic modality, pharmacists need to understand the diagnosis, pathophysiology, and treatment of these diseases since patients may attempt self-care of these disorders, require education for proper medication use, or may rely on the pharmacist to help them manage their disease.
• ETIOLOGY/PATHOPHYSIOLOGY/EPIDEMIOLOGY
While both asthma and COPD have major small airway disease components with inflammation and increased airway resistance, they have different etiologies, characteristics, and outcomes (see Table 21.1).
| TABLE 21.1 | Differences Between Asthma and COPD |
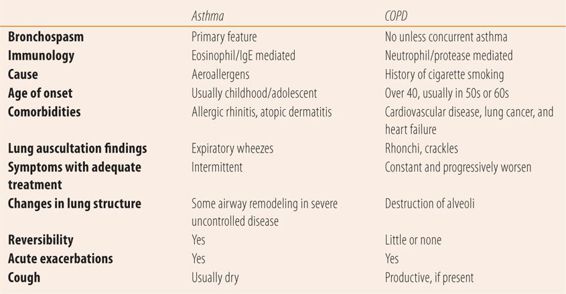
Asthma
Asthma affects patients of all ages. Over 25% of the 25.9 million patients with asthma are children. Asthma is a chronic inflammatory disease of the airways. The inflammation causes airway hyperresponsiveness to various stimuli, leading to bronchospasm, which manifests itself as coughing in the early morning hours, wheezing, breathlessness, and chest tightness. The inflammation also causes airway obstruction that limits airflow that can be reversed with treatment. Long-term untreated disease can lead to airway remodeling that may not be completely reversible. Initially, the pathogenesis was thought to be exclusively due to IgE-mediated reactions involving eosinophils. However, while eosinophilis and IgE are still important elements in the pathogenesis of asthma, over the last several years evidence has shown asthma to be a heterogeneous disease. Different patterns of inflammatory processes that involve multiple cellular mechanisms, result in different disease intensity and varying response to guideline-based treatments. Currently, the development of asthma is thought to be due to an interaction among innate immunity, a complex genetic component, and environmental factors, including respiratory syncytial virus, rhinovirus, and airborne allergens. Recent evidence also shows that this process impedes the normal epithelial cell barrier (like atopic dermatitis), which allows antigens to pass into local tissue to create the interaction between the three major pathogenic components. In patients with stable, well-controlled asthma, exacerbations are mostly due to rhinovirus infections. Allergen concentration, suboptimal medication adherence, and other triggering substances or medications are also responsible for exacerbations in poorly controlled asthma.
COPD
While airway inflammation is a major component of COPD, the process is totally different than with asthma. Cigarette smoke and other toxins, e.g., indoor (smoke from burning wood) and outdoor air pollution are the primary causative agents in COPD. These noxious toxins stimulate the release of proteases from neutrophils, which cause alveolar wall destruction (emphysema), mucous hypersecretion (chronic bronchitis), and fibrosis of lung tissue. This process accelerates the normal age-related annual rate of loss of lung function by threefold. Unfortunately, the lung damage is irreversible. Even when exposure to the noxious substance (cigarette smoke) ceases, the accelerated loss of lung function only returns to normal age-related rates of decline. Therefore, in the patient who quits smoking after being diagnosed with COPD, the disease will continue to worsen albeit at a slower, normal rate rather than the accelerated rate induced by smoking. There is a genetic component since only 25% of heavy smokers go on to develop COPD. Historically, COPD occurred primarily in men, because they more frequently smoked than women. Today, with the rise in the number of women who smoke, the incidence of COPD is roughly equally divided between men and women. In addition to losing lung function, exacerbations of COPD are both a clinical and economic burden. Exacerbations of COPD are defined as increased dyspnea for two consecutive days. Triggers for exacerbation include bacterial and viral respiratory tract infections, environmental pollutants, and unknown causes. Some patients have asthma in addition to COPD (asthmatics who smoke) and treatment of these patients involves anti-inflammatory drug therapy used in asthma patients.
• DIAGNOSIS
Spirometry
Spirometry, also known as pulmonary function testing (PFTs), is the major objective measurement of lung function used in the diagnosis of asthma and COPD. Portable instruments are available for office use and procedures have long been standardized. The patient takes a deep breath and blows into the machine as hard and fast as they can for as long as they can. This is repeated several times and if the results are similar, the machine calculates several values. Once initial values are established, two puffs of a short-acting bronchodilator are administered and PFTs are repeated 10 to 20 minutes later. The most important values are the forced expiratory volume during the first second (FEV1) and the forced vital capacity (FVC), the total amount of air exhaled during the test. Both values vary with gender, age, and height. Both FEV1 and FVC peak at about 20 to 25 years of age and at approximately 35 years of age begin to slowly decline. The normal annual rate of loss increases with age starting out at 10 to 15 cc/year at 35 and increasing to nearly 60 cc/year by age 70. Changes between the prebronchodilator and postbronchodilator values determine the reversibility and are used to confirm the diagnosis of concurrent asthma.
Asthma
A careful history is required as part of a workup for asthma. Key symptoms include recurrent bouts of wheezing, difficulty breathing, and chest tightness. Some patients’ symptoms may manifest themselves primarily as cough during exercise or between 2 and 6 am, when lung vital capacity is at its lowest due to circadian variation. Identifying situations that precipitate or aggravate symptoms provides important diagnostic clues. Exercise, viral infection, exposure to inhaled allergens, e.g., cat or dog dander, pollen, etc., all can trigger or worsen symptoms. Symptoms that occur or worsen at night, awakening the patient, are common in asthma. Coughing episodes between 2 and 6 am are almost diagnostic in children. Finally, a personal history of allergic rhinitis or atopic dermatitis is a common finding in patients with asthma, as is a history of asthma, allergic rhinitis, and atopic dermatitis in a direct blood relative. Many patients with asthma also have allergic rhinitis and symptoms of asthma may worsen with exacerbation of allergic rhinitis symptoms. Findings during physical examination can include expiratory wheezing during auscultation of the lungs. However, many times the patients have a normal chest examination. Careful examination of the cubital and popliteal fossa (the hollow of the elbow and knee respectively) for the eczematous rash of atopic dermatitis and an ENT examination for signs of allergic rhinitis (e.g., allergic shiners, nasal crease, Dennie’s lines, and pale boggy nasal mucosa, with a thin watery nasal discharge) are important. Lung function needs to be evaluated by spirometry both pre- and postbronchodilator. After baseline values are established, the patient is given two puffs of albuterol by metered dose inhaler (MDI). FEV1 values should increase by at least 12% 10 to 20 minutes after bronchodilator administration to establish the reversibility of bronchoconstriction that is diagnostic of asthma. Fractional nitric oxide (NO) concentration in exhaled breath (FENO) is also used as an aid to diagnosis. In classical asthma, sputum eosinophilia and IgE are markers of airway inflammation. Since eosinophils cause the production of NO, the degree of inflammation can be assessed by the amount of NO exhaled. Greater than 50 parts/billion (ppb) (>35 ppb in children) is diagnostic of asthma. However, since the nature of asthma is heterogeneous regarding pathogenesis, some patients will have low NO levels because their asthma is not the predominant type (eosinophilia, plus IgE mediated). However, elevated FENO levels will predict the likelihood of response to inhaled corticosteroids and can be used for monitoring therapy effectiveness, adjusting doses, confirming medication adherence problems, and identifying suboptimal inhaler technique.
COPD
In patients over 40 years of age, COPD should be suspected with any of the following: history of difficulty breathing (dyspnea) that has tended to worsen over time or worsen with exercise, chronic cough with productive sputum, history of cigarette smoking, or a family history of COPD. However, there are exceptions to the classical pattern listed above. Some patients will not identify difficulty in breathing, but will instead talk about not being able to walk very far without their legs getting tired or heavy. Similarly, a cough may be absent, intermittent or non-productive or be dismissed as an expected consequence of smoking. Patients may have had no exposure to cigarettes, but have regular exposure to wood smoke or industrial or occupational pollutants. Patients need not be current smokers, but usually have a long history of cigarette smoking, which greatly accelerates lung function loss. A history concerning the frequency of possible COPD exacerbations needs to be done. Unfortunately, patients may not recognize them, so questioning should include a history of hospitalizations for respiratory disorders, treatment of lower respiratory disorders with antibiotics, corticosteroids or inhalers, and colds that “go to the chest” and/or “last forever.” Physical examination in many cases is normal. In patients with a productive cough, rhonchi or crackles can be heard on auscultation of the lungs. Rhonchi are heard in both inspiration and expiration. They have a low-pitched snoring, gurgling, rattle-like quality. Rhonchi represent secretions/mucous in large airways, and leaving the stethoscope in place and having the patient cough will cause the rhonchi to disappear or drastically change. In addition, breath sounds may be decreased due to destruction of lung tissue and air trapping. In patients with more advanced COPD, the patient will have tachypnea and tachycardia and the pulse oximetry may be less than 92% on room air. In patients with severe and long-standing disease, patients will not be able to speak in complete sentences without taking a breath and may have unusually prominent sternocleidomastoid muscles (in the neck) during each inspiration. Spirometry again establishes airflow limitations and the diagnosis. In COPD, postbronchodilator the FEV1/FVC is less than 0.70 and the lower the FEV1, the more severe the COPD. There are standardized questionnaires to measure functional dyspnea severity, such as the Modified Medical Research Council scale (mMRC) and the COPD Assessment Test (CAT). The mMRC uses the degree of breathlessness related to physical activities such as dressing and walking uphill. The CAT uses a 6-point scale and 8 statements regarding the extent of cough, congestion, tightness, breathlessness with exercise, sleep quality, and energy levels. Both are more predictive than FEV1 alone in predicting mortality.
• CLASSIFICATION
Both asthma and COPD have classification systems based on severity of symptoms and spirometry values that are primarily intended to guide therapy.
Asthma
The asthma classification system distinguishes between intermittent asthma and persistent asthma based on symptoms, nighttime awakenings, interference with normal activity, and the frequency of use of short-acting β-adrenergic inhalers (SABA) and FEV1 using a peak flowmeter. See the National Asthma Education and Prevention Program’s Expert Panel Report 3 (EPR-3) Guidelines for the Diagnosis and Management of Asthma for specific details by age. Intermittent asthma is characterized by no more than two episodes of symptoms/week, no more than two episodes/month of nighttime awakenings, SABA use no more than two times/week, no interference with normal activities, and a normal FEV1 between exacerbations. Persistent asthma is divided into mild, moderate, and severe based on those same parameters. Each level of severity is tied to a specific intensity of treatment.
COPD
The classification system for COPD is international and is composed of three parts: objective airflow limitations, symptoms, and risk of future exacerbations. The first is based on postbronchodilator FEV1 values with four levels of severity in patients with FEV1/FVC <0.70, with mild or level one having an FEV1 greater than or equal to 80% of predicted value, ranging to the fourth level where the FEV1 is less than 30% of predicted value. Second is a measure of the severity of functional dyspnea using the mMRC or CAT. The third part is the frequency of COPD exacerbations/year. These three parts are combined into an ABCD classification system that is tied to recommended treatment regimens. For specific details, see the Global Initiative for Chronic Obstructive Lung Disease’s (GOLD) Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease: GOLD Executive Summary.
• COMPLICATIONS
Asthma
Over 3000 people die every year from asthma, and in 7000 additional deaths asthma was a contributing factor. The primary complication of asthma is respiratory failure from acute asthma attacks. The goal of asthma treatment is to prevent these acute attacks by controlling the inflammation with inhaled corticosteroids or drugs that interfere with the effects of leukotrienes and other mediators. Acute asthma attacks due to poorly controlled asthma were the cause of nearly 500,000 hospitalizations and 20 million emergency room visits in 2012. Viral respiratory tract infections are the most common precipitant of an acute asthma attack. Other common causes include exposure to known allergens or triggers of asthma attacks, inadequate use or dose of inhaled corticosteroids, lack of objective monitoring criteria (lack of an asthma action plan). Some patients, especially those with severe asthma, may have a blunted perception of worsening bronchospasm. Peak expiratory flow (PEFR) monitoring with a handheld peak flowmeter, which approximates FEV1, helps those patients better assess the presence of acute asthma attacks. Most acute asthma attacks respond to standard treatments of intense bronchodilation and hydration. When an acute attack fails to respond to standard therapy, the term status asthmaticus may be used. This is the most difficult form of asthma to treat. Table 21.2 lists subjective and objective parameters that indicate severe acute asthma attacks similar to status asthmaticus.
| TABLE 21.2 | Danger Signs During Acute Asthma Attacks |

COPD
The primary cause of death in COPD is respiratory failure either due to chronic deterioration or acute respiratory failure brought on by bacterial or viral pneumonia, which markedly reduces the already severely limited capacity of the lungs. In addition, the years of smoking predisposes patients to cardiovascular disease, e.g., coronary artery disease and stroke. In addition, severe hypoxia poses a risk for fatal cardiac dysrhythmias. Finally, the smoking markedly increases the risk for lung cancer. Unfortunately, by the time of COPD diagnosis most patients are past middle age and already have symptoms due to the threefold rate of loss of pulmonary function due to the smoking-induced inflammatory process. While the rate of loss of pulmonary function returns to the normal rate after smoking cessation, lung function continues to deteriorate, but at a slower normal rate. Unfortunately, treatment cannot reverse the damage in susceptible people. For people with the most severe disease, Group C and D (GOLD 3 and 4), there are several integrated disease severity indexes that can be used to predict 3-year mortality risk. The first is the BODE index. Parameters of the BODE index include BMI, obstruction of airflow (FEV1), dyspnea measured via the mMRC, and exercise capacity (6-minute walk test). The second is simpler, the ADO index, where age, dyspnea, and obstruction of airflow (FEV1) are used.
• INITIAL VISIT
For both asthma and COPD, the initial visit workup consists of several parts. First the diagnosis needs to be confirmed (see “Diagnosis”). Second, the severity of the disease needs to be classified to determine appropriate initial therapy, which entails a history of exacerbations for COPD and aggravating or precipitating factors in asthma. Third, the psychosociological aspects of the disease and support structure for treatment and control of the disease needs to be ascertained, including any potential adherence barriers or issues. Finally, a history and physical examination should be done to ascertain the presence of comorbid conditions that may impact overall health and adjustment of therapy for asthma or COPD, such as cardiovascular disease in COPD or atopic dermatitis and allergic rhinitis in asthma. See Key References 1 and 10 for specific details of initial visit workups.
• FOLLOW-UP VISITS
Like any chronic disease, follow-up visits for asthma and COPD are an important part of optimizing disease control and preventing complications. In asthma, ideally we want to prevent acute attacks and prevent symptoms that may indicate uncontrolled bronchospasm. However, due to the difficulty posed by normal variation of exposure to aeroallergens and other triggers of asthma symptoms, national guidelines recommended a more pragmatic approach to define well-controlled asthma. Five basic parameters are used including frequency of symptoms (cough, chest tightness, etc.), nighttime awakenings, use of rescue inhalers such as albuterol, FEV1 (PEFR), and limitations on activities caused by asthma. Well-controlled asthma is defined as symptoms no more than 2 days per week, nighttime awakenings no more than two times per month, no limitations of activities due to asthma, use of rescue inhalers no more than 2 days per week, and a peak expiratory flow reading (PEFR) of more than 80% of predicted value or in more severe forms of asthma the patient’s personal best (when they are well controlled). Other organizations have tried to simplify that definition by using a “rule of 2s.” Asthma is not well controlled if you use a rescue inhaler more than two times a week, have more than two episodes of nighttime symptoms per month, or refill the rescue inhaler more than two times a year. There are self-administered validated instruments that are based on the definition of well controlled that can be used to assist in assessment, including the Asthma Control Test (ACT), the Asthma Control Questionnaire (ACQ), and the Asthma Therapy Assessment Questionnaire (ATAQ). A comprehensive list of subjective and objective data that needs to be collected at each follow-up visit can be found in Table 21.3. Obviously any responses that indicate poor control need to be probed in depth, including when, why, and what, to get specific details that may aid in determining changes in pharmacological or nonpharmacological therapy.
| TABLE 21.3 | Follow-Up Visit Parameters for Asthma |


One of the psychological problems in patients with asthma is the anguish of being unable to breath and the sense of no control over symptoms when they occur. Also patients may be unsure what symptoms are an indication of a need for additional care. In the past, these three factors caused visits to the emergency room that did not match the degree of clinical impairment. Eventually asthma action plans were developed and are now required for each patient. The purpose of the asthma action plan is to allow the self-treatment of minor attacks with asthma medication and provide criteria for assessing the severity of the attack, to determine the need for an emergency room visit or call to the physician. The plan is based on PEFR readings. Patients are told to obtain a PEFR upon awakening (because FVC is at its lowest between 2 and 6 AM) and whenever they notice asthma symptoms. Generally, if the PEFR is 80% of predicted/personal best or higher (in the “green zone” or good zone), then nothing additional needs to be done other than continuing to take their long-term control medicine and avoiding triggers. If the readings are in the “yellow zone” (50% to 80% of predicted/personal best), specific directions on using short-acting bronchodilators are indicated. Also, patients are instructed to wait for a certain amount of time to allow the bronchodilator to work, then repeat the PEFR. If the PEFR is back in the green zone (>80%), then nothing else needs to be done. If after treatment the PEFR is still in the yellow zone, patient-specific instructions for the next step are listed, e.g., repeat bronchodilator treatment, go to the emergency room, or call the physician. If any reading is less than 50% of predicted or personal best, it is in the “red zone.” The asthma action plan has instructions for patients in the red zone to go to the emergency room immediately. The directions for what to do if the patient has a yellow zone reading is individualized for each patient. Also, the boundaries of PEFR for each zone can be individualized based on the severity of the disease and the sophistication and comfort of the patient with self-care of acute asthma attacks. Self-management of asthma symptoms using an asthma action plan improves disease control and medication adherence. In addition, asthma action plans significantly reduce the frequency of emergency room visits, and markedly enhance the patient’s sense of control of their asthma and satisfaction with asthma care.
One thing that should be checked regularly at each routine visit for both asthma and COPD is proper inhaler use. Multiple studies show that more than 90% of patients make at least one error in technique even though they felt they knew how to correctly use their inhalers. Unfortunately, each type of inhaler has its own specific technique. If unsure about proper technique, there are excellent videos available via the Internet. National Jewish Health, world renown for their care of patients with respiratory disease, has an excellent set of instructional videos, for each type of device, posted on You-Tube and available on their web site (http://www.nationaljewish.org/healthinfo/medications/lung-diseases/devices/instructional-videos).
In COPD, the main goal is to relieve dyspnea as much as possible with long-acting bronchodilators and oxygen, and to reduce the numbers of exacerbations. Since smoking is the primary cause of COPD, smoking cessation is the only treatment that will slow the rate of loss of lung function. In nonsmokers, the avoidance of the noxious substances functions like smoking cessation. Questions regarding changes in exercise capacity or ability to do routine activities are a measure of the severity of dyspnea. A comprehensive list of subjective and objective data that needs to be collected at each follow-up visit for COPD can be found in Table 21.4. While not suggested by guidelines, some providers use COPD action plans that are similar in structure, design and purpose to asthma action plans. Signs and symptoms of COPD exacerbations are acute in nature and differ from normal variation and include increased dyspnea, increased sputum volume, worsening tachycardia/tachypnea, and/or increased sputum purulence. In severe forms of both asthma and COPD, hypoxia from the disease can cause several changes in breathing patterns. Signs include an elevated respiratory rate (>20 breaths/minute), inability to speak in complete sentences without taking a breath, and the contraction of ancillary muscles of respiration in the neck when breathing. In severe COPD, the sternocleidomastoid muscles enlarge due to regular use and in slender patients, protrude even when not taking a breath. Finally, pulse oximetry values less than 91% on room air are abnormally low, but are not uncommon.
| TABLE 21.4 | Follow-Up Visit Parameters for COPD |


• KEY REFERENCES
1. Anon. Expert Panel Report 3 (EPR-3): guidelines for the diagnosis and treatment of asthma summary report 2007. J Allergy Clin Immunol. 2007;120:S93-S138.
2. Self TH, Wallace JL, George CM, Howard-Thompson A, Schrock SD. Inhalation therapy: help patients avoid these mistakes. J Fam Pract. 2011;60:714-721.
3. Sims MW. Aerosol therapy for obstructive lung diseases. Chest. 2011;140:781-788.
4. Dweik RA, Boggs PB, Erzurum SC, et al. An official ATS clinical practice guidline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011;184:602-615.
5. Higgins JC. The “crashing asthmatic.” Am Fam Physician. 2003;67:997-1004.
6. Liou TG, Kanner RE. Spirometry. Clin Rev Allergy Immunol. 2009;37:137-152.
7. Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:1005-1012.
8. Puhan MA, Garcia-Aymerich J, Frey M, et al. Expansion of the prognostic assessment of patients with chronic obstructive pulmonary disease: the updated BODE index and the ADO index. Lancet. 2009;374:704-711.
9. Rosenberg SR, Kalhan R. An integrated approach to the medical treatment of chronic obstructive pulmonary disease. Med Clin North Am. 2012;96:811-826.
10. Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187:347-365.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



