Fig. 13.1
Sagittal section of the anatomy of the anal canal
Proximally these extend as primary rectal foldings and are separated and covered by the rectal columns of Morgagni [4]. Caudal to this the anal canal is lined by stratified squamous epithelium. The anal cushions are made up of mucosa, submucosa, fibroelastic connective tissue, smooth muscle and blood vessels.
Function of Anal Cushions
The function of the anal cushions has been the topic of much interest and debate. The fact that they can vary their size, given their vascular components, suggests that they have a role in continence. Hence, if we propose that anal cushions are physiological, then only when they become pathological should they be referred to as haemorrhoids.
Studies suggest that the internal sphincter alone cannot completely close the anal canal even during periods of maximal contraction. Lestar et al. demonstrated that the anal cushions have to fill up an intrasphincteric gap of at least 7–8 mm in diameter [5]. Vascular filling contributes to 15–20 % of resting anal pressure as evident from electrophysiological studies [6]. Anal cushions probably provide a plug to maintain continence, and indeed haemorrhoidectomy has been shown to impair continence to infused saline [7].
Mechanisms by which anal cushions facilitate defecation have been studied with much interest. Passive pressure from stool may contribute. In addition active mechanisms include (1) anal dilatation which in turn reduces the height of anal cushions and (2) contraction of the subepithelial smooth muscle layers and the conjoint longitudinal layer resulting in displacement of the anal cushions. The conjoint longitudinal layer has both voluntary and involuntary components that function as a complex to control anal action during defecation.
Anatomy of Haemorrhoids
With age, the sphincter complex changes. The smooth muscle layer becomes replaced by connective tissue. In addition the submucosa thickens and the connective issue becomes looser and more fragmented [8]. The sphincter complex looses strength and the anal cushions are less protected from the forces placed upon them. The cushions become more vulnerable to trauma and migrate downwards with the effect of gravity, and the loss of muscular elements of the submucosa may reduce contractility and strength of the walls of the vascular components rendering them more likely to bleed or thrombose. Figure 13.2 illustrates anatomical differences between first-, second- and third-degree haemorrhoids.
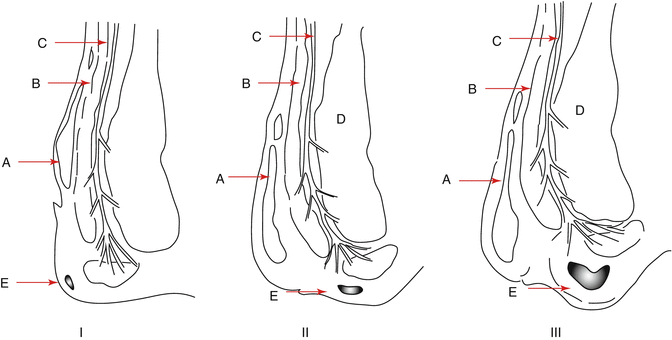

Fig. 13.2
Classification of haemorrhoids: sagittal section of the anal canal demonstrating the descent of the anal cushions. A Submucosal plexus, B Internal anal sphincter, C Longitudinal muscle, D External anal sphincter, E Anal cushion
Histological analysis of haemorrhoidectomy specimens reveals squamous metaplasia and dense capillary vasculature. There is reduced collagen to protein ratio and a reduced type I to type III collagen ratio. Hence, there may be a disorder of collagen metabolism that needs attention [9].
Blood Supply
Arteries
The arterial supply of the haemorrhoidal plexus is from the superior, middle and inferior haemorrhoidal arteries. The superior haemorrhoidal artery is a continuation of the inferior mesenteric artery and descends into the pelvis in the sigmoid mesocolon. It divides into two further branches on either side of the rectum at the level of the third sacral vertebra. Further smaller branches traverse the rectal wall at the submucosal level to supply the internal sphincter and anastomose with branches of the middle and inferior haemorrhoidal arteries. The middle rectal artery arises from the anterior trunk of the internal iliac artery with the inferior vesical artery, whilst the inferior rectal artery arises above the ischial tuberosity from the internal pudendal artery, which is itself a branch of the internal iliac artery.
This rich anastomosis makes rectal ischaemia uncommon and bleeding from haemorrhoids a distinct problem.
Veins
The venous return of the haemorrhoidal plexus follows the arterial pattern. The superior rectal vein drains into the inferior mesenteric vein, which itself drains into the portal vein. The middle and inferior haemorrhoidal veins drain directly to the internal iliac veins and via the pudendal vein to the internal iliac vein, respectively. They form a site of portosystemic venous anastomosis which is relevant in patients with portal hypertension. Haemorrhoids in these patients are far rarer than true rectal varices. This portosystemic anastomosis is also the likely route of rare cases of mesenteric or portal pyaemia, portal thrombosis and sepsis and even liver abscesses seen after injection sclerotherapy, banding or ligation of haemorrhoids [10].
Lymphatic Drainage
The upper half of the rectum has lymphatic drainage which follows the superior haemorrhoidal vessels through pararectal nodes to nodes in the lower sigmoid mesocolon and along the IMA [11].
The lymphatics of the lower half of the rectum and the anal canal proximal to the dentate line accompany the middle rectal vessels to the internal iliac nodes.
Lymphatics of the anal canal below the dentate line drain into superficial inguinal nodes.
Innervation
The inferior rectal nerve (S2, S3) and to a lesser degree the perineal nerve (S4), both branches of the pudendal nerve (S2, S3), provide somatic innervation of the anal canal below the dentate line. This innervation is responsible for the pain felt during thrombosis of external haemorrhoids as well as pain after surgical excision.
Above the dentate line, there is an absence of somatic innervation and only the presence of sensation provided by visceral afferent fibres which join the inferior hypogastric plexus. Hence, internal haemorrhoids are only painful if acutely prolapsed or thrombosed.
Aetiology
Theories of Pathogenesis
Varicose Vein Theory
Venous dilatations within the haemorrhoidal specimens raised the concept that haemorrhoids were the result of dilatation and weakness of veins. The absence of valves in the portal system along with the upright posture of human beings was thought to result in increasing resting venous pressure. However, this theory was disputed by the discovery of portosystemic anastomosis which allows dissipation of any increased pressure [12].
Vascular Hyperplasia Theory
Haemorrhoidal tissue was believed to be cavernous tissue much like corpus cavernosum. Arteriovenous communications suggested that erectile properties of this tissue could contribute to the continence mechanism. Hence, it was referred to as corpus cavernosum recti by Virchow and Allingham and quoted by Thomson in 1975 [3]. This theory was disputed on histological grounds by Thomson who believed that the bleeding from haemorrhoids arose from ‘capillaries in the lamina propria’ rather than the venous dilatation. Though this theory was thrown into disrepute in the long run, it did highlight the arteriovenous connections, as opposed to solely venous communications, and explained the nature of the bright red bleeding from haemorrhoids.
Infection/Inflammation Theory
Quanu and Hartmans in the nineteenth century suggested that repeated trauma by defecation resulted in infections and a consequent weakness of the wall of the haemorrhoidal vein, as stated by McGivney in 1967 [13]. However, a distinct lack of histological evidence made this theory unlikely. Further anatomical dissections revealed that these so-called venous dilatations were physiological.
Morgagni in the seventeenth century proposed that the effects of the erect posture could be maintained, until affected by infection, in which case haemorrhoids result.
With further anatomical and histological developments into haemorrhoidal aetiology, these theories were dismissed by Jackson and Robertson in the American proctologic society meeting in 1964. These modern ideas were supported by the fact that haemorrhoids histologically demonstrate venous thickening as opposed to thinning [14].
Sliding Anal Lining Theory
Detailed observation of microscopic sections of haemorrhoids from over 4,000 patients in Tennessee by Gass and Adam demonstrated loose and fragmented submucosal collagenous and connective tissue stroma as the fundamental early lesion in haemorrhoidal disease [15]. The vein walls showed a compensatory thickening rather than thinning as proposed in the varicose vein theory. This paved the way for further anatomical studies demonstrating age-related disruption and thickening of the collagen network which provides support for the anal cushions. This results in eventual sliding of anal cushions and explains the progressive nature of haemorrhoids with age.
Predisposing Factors
Analysis of predisposing factors adds to our knowledge of why haemorrhoids occur. Though many have been identified as risk factors, evidence for these remains limited (Table 13.1).
Table 13.1
Symptoms unlikely to be attributed to haemorrhoids alone
Presentation | Alternate diagnosis |
|---|---|
Change in bowel habit | Inflammatory bowel disease, malignancy |
Faecal discharge/seepage | Rectal prolapse, neoplasm, polyp |
Iron deficiency anaemia | Colorectal cancer (right > left) |
Diet and Stool Consistency
Many studies have analysed the connection between constipation and haemorrhoids. Early attempts on disease management concentrated on increasing fibre intake and normalising bowel habit. Parks in 1956 suggested that the hard faecal matter resulted in venous obstruction and consequent engorgement of the haemorrhoids [16].
However, a large epidemiological study by Johnson et al. in 1990 demonstrated that constipation is not associated with haemorrhoidal disease, and indeed later studies suggest that conversely there might be an association between diarrhoea and haemorrhoids [17].
Further work by Gibbons et al. has cast doubt on the theory that constipation is a contributing factor to the development of haemorrhoids. Their group of constipated women had normal anal pressure and compliance profiles [18].
Hancock did not demonstrate a difference in bowel frequency in patients with haemorrhoids but did demonstrate that patients with prolapsing haemorrhoids were more likely to strain that controls. Whether this was cause or effect of haemorrhoids remains unclear [19].
Defecatory Position
It has been postulated that protracted time sitting or squatting on the lavatory with an unsupported perineum is associated with the development of haemorrhoids [3]. However, scientific evidence for this theory remains limited.
Genetics
Though a positive family history is common in patients with haemorrhoids [20], no specific genetic breakthrough has been made as yet. Family history may be secondary to common cultural and diet-related factors as opposed to genetics alone. In addition there may be bias in parents of the same sex who are more likely to report perianal disease than parents of the opposite sex.
Abdominal and Pelvic Causes
Physiological causes of increased intra-abdominal pressure include pregnancy. Pathological causes include uterine fibroids, abdominal tumours and ovarian cysts. Haemorrhoids may be associated with prostatism and hernias, both of which are associated with abdominal straining, although the association with hernias may be collagen related. Pressure from pelvic masses may cause engorgement of haemorrhoids by obstructing venous return.
Functional Abnormalities of Haemorrhoids
Despite advances in anatomy, and many studies on aetiology of haemorrhoids, the question remains: if anal cushions are part of normal human anatomy, what makes them transform into haemorrhoids? Advances in physiological studies may add further answers. We analyse the evidence suggesting increased anal pressure in patients with haemorrhoids below.
Increased Anal Pressure
Anorectal physiology studies demonstrate increased resting anal pressure in patients with haemorrhoidal disease. Whether this contributes to or is the result of haemorrhoids remains unclear. Whilst an increased sphincter tone may result in hypertrophy of anal cushions, constant distension of anal cushions may itself result in an increased resting anal pressure. Arabi et al. demonstrated significantly higher anal pressures in patients with haemorrhoids, but this was reserved for young male patients [21]. Anal pressures returned to normal in patients 3 months after surgical haemorrhoidectomy, yet normal resting pressures are not affected by haemorrhoidectomy [22]. Only partial reduction in pressure is noted in patients with rubber band ligation [23]. As pressures return to normal after haemorrhoid surgery, it could be argued that the increased resting anal pressures are more likely to be the result of haemorrhoidal disease rather than the cause of it.
Not all patients’ haemorrhoids exhibit raised anal resting pressures. This implies that haemorrhoidal disease is multifactorial in aetiology [24]. Patients with anal fissures have high anal pressure profiles but do not necessarily develop prolapsing haemorrhoids [21]. In addition, the prevalence of haemorrhoids increases with age, whereas the anal sphincter pressure declines with age.
Studies have investigated the predilection of female gender and elevated anal pressures causing haemorrhoids. Though some reports encourage this notion [23, 24], most studies do not reach statistical significance to allow us to establish a clear connection.
External Anal Sphincter
The external sphincter of patients with haemorrhoids demonstrates muscle hypertrophy, possibly as a result of increased contraction. This has been demonstrated on EMG recording and may contribute to the raised anal canal pressure. Using single-fibre EMG, Bruck et al. demonstrated increased external sphincter fibre density in haemorrhoids patients, implying reinnervation after denervation [25]. Pudendal nerve terminal motor latency was also prolonged implying impaired conduction.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


