Glycogen-Rich Carcinoma
FREDERICK C. KOERNER
Carcinomas that accumulate abundant glycogen arise in many organs, including the lungs, endometrium, cervix, ovary, and salivary glands.1 Extraction of the water-soluble glycogen during histologic processing causes the cytoplasm to become vacuolated or completely clear in conventional hematoxylin and eosin (H&E)-stained sections, and this phenomenon has led pathologists to designate such tumors as clear cell carcinomas. In 1981, Hull et al.2 described an in situ and invasive mammary carcinoma composed of cells with clear cytoplasm and proposed the diagnosis of glycogen-rich clear cell carcinoma of the breast for this variant of mammary duct carcinoma. These writers did not establish criteria for this diagnosis, nor did they estimate the frequency of the lesion; however, subsequent observers have commented on both points. Fisher et al.3 required that 50% or more of the cells contain “optically clear cytoplasm and, usually, centrally placed nuclei” to make the diagnosis of glycogen-rich clear cell carcinoma. Having done so, the authors classified 45 of 1,555 breast carcinomas (3%) as glycogen-rich clear cell carcinoma. Kuroda et al.4 used the same threshold and discovered 20 glycogen-rich clear cell carcinomas in a group of 723 primary breast carcinomas (2.7%). Toikkanen and Joensuu5 required that 90% of the carcinoma cells contain clear cytoplasm to make the diagnosis of glycogen-rich clear cell carcinoma. They found 6 of 439 breast carcinomas (1.4%) met their criteria for this diagnosis. Hull and Warfel6 did not specify their diagnostic criteria, but they regarded only 9 of 936 breast carcinomas (1%) as glycogen-rich clear cell carcinomas. These findings indicate that glycogen-rich clear cell carcinoma is an exceedingly rare form of primary breast carcinoma. Fewer than 150 well-documented examples have been described since the first case was reported in 1981.
CLINICAL PRESENTATION
The patients, whose ages ranged from 32 to 81 years,7 presented with a mass accompanied by skin dimpling, nipple retraction, or pain in some cases. Both in situ and invasive lesions may be detected by mammography2,8,9,10,11,12 and sonography.2,11 The imaging studies reveal an irregular spiculated mass8,10,11,13 that may contain calcifications.10,11,13
GROSS PATHOLOGY
Glycogen-rich carcinomas resemble conventional breast carcinomas to the unaided eye. Distinctive macroscopic features have not been recorded. Most tumors measured between 2 and 5 cm; the largest spanned “about 15 cm.”13 In one series, the mean size was 3 cm.7 Observers have described the masses as “brownish pink-gray or whitish-gray.”7 The carcinoma can form multifocal or multicentric masses, and macroscopically evident involvement of the skin occurred in several cases.7,14 The seminal authors could appreciate an in situ papillary component during macroscopic examination.2
MICROSCOPIC PATHOLOGY
Glycogen-rich clear cell carcinomas have basic structural features of ductal carcinoma in situ (DCIS) alone or of DCIS and infiltrating duct carcinoma. The intraductal component can grow in papillary, solid, cribriform, micropapillary, and “intracystic” patterns. Cytoplasmic clearing appears most evident in solid areas that also exhibit moderate nuclear atypia (Fig. 28.1). The cells in cribriform and micropapillary regions usually have low-grade atypia and less often appear water-clear. The neoplastic cells can undergo focal necrosis, but abundant, comedolike necrosis associated with high-grade nuclear atypia does not occur commonly.9 In most cases, one can detect small regions in which the cells contain eosinophilic and granular cytoplasm or exhibit other clear-cut apocrine features. The invasive component usually exhibits the histologic growth pattern of a conventional invasive duct carcinoma, but it can also display the patterns seen in lobular, medullary, and tubular carcinomas.3 The tumor cells typically form cords, solid nests, or papillary structures (Fig. 28.2). The formation of ductular or tubular structures occurs only rarely. The cells exhibit sharply defined borders and polygonal rather than rounded contours. The cytoplasm is clear or, less often, finely granular or foamy. Like the noninvasive component, the invasive carcinoma sometimes contains foci in which the cells have eosinophilic and granular cytoplasm that suggests an apocrine nature, and these cells often form a continuum with the clear cells. Observers have noted PAS-positive, diastase-resistant, intracytoplasmic hyalin droplets in rare cases.9 The nuclei appear hyperchromatic and
sometimes contain clumped chromatin and nucleoli. Mitotic figures are easily identified in most cases. One group15 found as many as 70 mitotic figures per 10 high-power fields. Patches of necrosis often occur in large tumors. A linear pattern consisting of strands of cells resembling invasive lobular carcinoma may be seen, and glycogen-rich variants of tubular, medullary, and endocrine carcinomas have been described.3,7,16,17 The carcinoma cells can invade lymphatic vessels and nerves. The histologic appearance of the primary tumor is duplicated in the metastases, which also contain abundant glycogen.6
sometimes contain clumped chromatin and nucleoli. Mitotic figures are easily identified in most cases. One group15 found as many as 70 mitotic figures per 10 high-power fields. Patches of necrosis often occur in large tumors. A linear pattern consisting of strands of cells resembling invasive lobular carcinoma may be seen, and glycogen-rich variants of tubular, medullary, and endocrine carcinomas have been described.3,7,16,17 The carcinoma cells can invade lymphatic vessels and nerves. The histologic appearance of the primary tumor is duplicated in the metastases, which also contain abundant glycogen.6
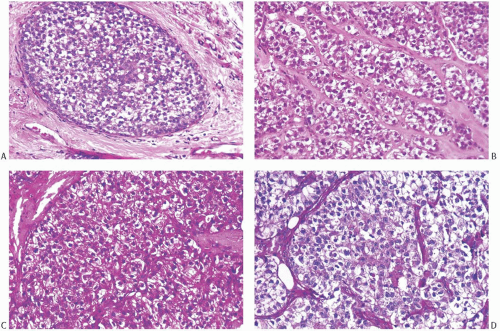 FIG. 28.1. Glycogen-rich carcinoma. A,B: DCIS and invasive carcinoma composed of cells with clear cytoplasm and small, dark, punctate nuclei. C: The tumor is strongly positive with the PAS reaction. D: After treatment with diastase, PAS reactivity is almost entirely abolished. The same pattern of PAS staining occurred in the intraductal carcinoma. |
Differential Diagnosis
The differential diagnosis of glycogen-rich carcinoma includes benign and malignant mammary and extramammary neoplasms. The clear cell type of hidradenoma (eccrine acrospiroma) shares the presence of many glycogen-laden clear cells with glycogen-rich carcinoma. However, hidradenomas are centered in the dermis, they have well-defined, smooth contours, and they consist of uniform bland cells. Atypical and malignant hidradenomas pose greater challenges in differential diagnosis (see Chapter 42). Myoepithelial cells with clear cytoplasm can dominate uncommon examples of mammary adenomyoepithelioma. Detection of a second population consisting of glandular cells and immunohistochemical demonstration of proteins characteristic of myoepithelial cells will distinguish this tumor from glycogen-rich carcinoma.
The detection of glycogen in a mammary carcinoma does not establish the diagnosis of glycogen-rich clear cell carcinoma, for Fisher et al.3




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


