Gastrointestinal Tract
Rebecca Wilcox
Amy Noffsinger
Introduction: Gastrointestinal Frozen Section
There are only a few indications for intraoperative frozen section in the evaluation of gastrointestinal disorders. These include determination of the extent of spread of a tumor, the adequacy of tumor resection usually through evaluation of resection margins, and determination of a diagnosis in cases where an unusual finding is encountered at the time of surgery. Often, the question that the surgeon has regarding the specimen can be answered by careful gross examination. As a result, close communication between the surgeon and the pathologist is necessary for optimal specimen evaluation and patient care.
Frozen sections should never be performed on endoscopic biopsy specimens since the procedure may introduce artifacts into the specimen that may compromise the pathologist’s ability to make an accurate diagnosis, and the small amount of tissue present in such biopsies may be completely exhausted as a result of the procedure. In addition, a frozen section diagnosis rendered on an endoscopic biopsy specimen would not result in an immediate action on the part of the endoscopist solely on the basis of the histologic diagnosis. In addition, since small biopsy specimens can often be rapidly processed, a definite diagnosis may be provided in emergent cases within a few hours of the endoscopic procedure. In cases where possible perforation of a viscus is suspected as a result of the endoscopy, the decision regarding whether or not to take the patient to the operating room for treatment should be based on symptoms, not the results of frozen section, since these are often not definitive and can in some situations be misleading (Fig. 12.1).
Esophagus: Major Intraoperative Questions
There are only a few indications for frozen section evaluation of esophageal resection specimens. Most often, the pathologist is called upon to determine the adequacy of resection of an esophageal or gastroesophageal junction tumor. Less frequently, a definitive diagnosis for a submucosal or intramural mass lesion is requested.
Esophagus: Interpretation of the Frozen Section
Evaluation of Resection Margins
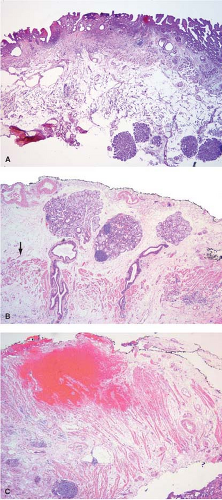
Esophagectomy or esophagogastrectomy specimens are indicated in patients with invasive squamous cell or adenocarcinoma of the esophagus or carcinoma of the gastroesophageal junction. In the past, esophageal resection was also undertaken for treatment of patients with high-grade dysplasia arising in the setting of Barrett esophagus. Currently, however, this radical approach to treatment of high-grade dysplasia is uncommon since less invasive endoscopic techniques have been shown to be of equal value in the treatment of preinvasive or early invasive Barrett-associated neoplasia (1,2,3) (Fig. 12.1).
In the case of esophagectomy or esophagogastrectomy, the pathologist is most often called upon to evaluate the adequacy of the resection margin. In such specimens, the gastric portion of the resection is usually sufficiently generous, and the tumor far enough away, that the distal margin can be evaluated grossly. In one study of 189 resections for esophageal or gastroesophageal junction carcinoma, a microscopically positive distal resection margin was found in 24 cases (4). In the patients with positive distal margins, the median distance of the distal margin from the tumor was 1 cm, with a range from 0.5 to 4.5 cm. The authors of this study suggest that a grossly measured distance of 5 cm between the tumor and the distal margin is sufficient to ensure an adequate resection.
The proximal esophageal margin is often closer to the tumor, and therefore, will more likely require frozen section for evaluation. For patients with squamous cell carcinomas of the esophagus, a proximal margin of at least 3 cm is recommended (5). It is important to note that esophageal
squamous cell carcinomas may be multifocal, and therefore, simple gross examination of the resection margin may not be adequate to determine if the margin is free of tumor (6,7). In addition, squamous dysplasia may occasionally be encountered at the margin of resection, but may not be identified grossly.
squamous cell carcinomas may be multifocal, and therefore, simple gross examination of the resection margin may not be adequate to determine if the margin is free of tumor (6,7). In addition, squamous dysplasia may occasionally be encountered at the margin of resection, but may not be identified grossly.
Studies suggest that 5 to 8 cm of grossly negative proximal esophagus should be resected in patients with gastroesophageal junction adenocarcinomas (8,9). Adenocarcinomas may on occasion extend proximally in the submucosa, leaving the overlying squamous epithelium intact (Fig. 12.2). Such submucosal invasion may extend for a considerable distance, with one reported case reaching upward for as much as 8 cm (10). Such submucosal extension is not grossly recognizable, and could therefore, be easily missed without frozen section evaluation. Some studies suggest that frozen section need only be performed in cases where the primary cancer is deeply invasive (T3 or T4), since the risk for a positive proximal resection margin is small in superficial lesions, but increases significantly with increasing depth of invasion by the primary tumor (11). However, an increasing number of patients are receiving endoscopic mucosal resections (EMR) for staging purposes prior to their esophagectomy. So called “buried Barrett” or “buried” carcinoma is known to occur underneath intact squamous mucosa upon re-epithelialization of the area of EMR making its
presence insidious in nature. Therefore, a gross only margin check of an esophagectomy may not be appropriate in patients who have had a history of previous EMR or ablation relatively near the area of resection margin.
presence insidious in nature. Therefore, a gross only margin check of an esophagectomy may not be appropriate in patients who have had a history of previous EMR or ablation relatively near the area of resection margin.
When margins for Barrett-associated adenocarcinoma are being evaluated, it is important to determine not just whether the margin is free of invasive carcinoma, but also that there is no Barrett-associated dysplasia present. It is also important to report whether nondysplastic Barrett mucosa is present at the margin since the goal of most Barrett-associated cancer resections is to remove not only the tumor, but also all of the Barrett mucosa which may be at risk for later neoplastic transformation.
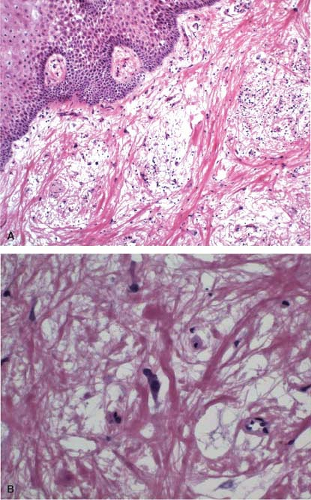
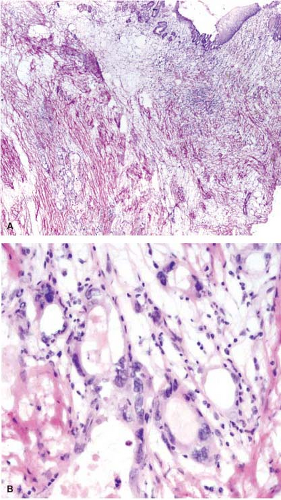
Preoperative radiation therapy is now used in majority of patients with esophageal carcinomas of both squamous and glandular origin. As a result, frozen sections performed for evaluation of the resection margin commonly show evidence of radiation injury. The fact that the patient has undergone such treatment is often not conveyed to the pathologist, and may result in misinterpretation of radiation atypia as neoplasia on frozen section (Fig. 12.3). In addition, foci of deep partially treated tumor may remain under re-epithelialized, non-neoplastic appearing mucosa (Fig. 12.4).
Both radiation- and chemotherapy-associated esophagitis result in large, bizarre-appearing squamous epithelial cells with increased cytoplasmic volume and enlarged nuclei. Bizarre (radiation) fibroblasts and vascular changes in the lamina propria are also common and suggest that radiation has been given preoperatively (Fig. 12.3). Epithelial hyperplasia develops in
an effort to re-epithelialize areas of erosion or ulceration. Mitotic figures may appear higher in the mucosa than normal, and the regenerating epithelium may show features simulating dysplasia.
an effort to re-epithelialize areas of erosion or ulceration. Mitotic figures may appear higher in the mucosa than normal, and the regenerating epithelium may show features simulating dysplasia.
Histologic examination in the later stages of radiation injury shows acanthosis, parakeratosis, hyperkeratosis, hyalinized blood vessels, submucosal and muscular fibrosis, and muscular degeneration. Fibrosis and degeneration affect the deeper esophageal tissues rather than the mucosa. The myenteric plexus becomes inflamed and fibrotic. The muscularis propria appears degenerated. Atypical fibroblasts may be embedded in dense collagenous tissue. Submucosal glands become atrophic, with acinar loss and dilatation, and inspissation of the ductular contents. Squamous metaplasia of the ducts of these glands and the presence of radiation-induced atypia may simulate invasive squamous cell carcinoma (Fig. 12.3).
Endoscopic Mucosal Resection Specimens
The pathologist may rarely be asked to determine adequacy of the margins of EMR specimens. EMR has become the first-line treatment for patients with Barrett-associated dysplasias and intramucosal carcinomas because the risk for nodal metastasis in these lesions is extremely low (12,13). The technique is minimally invasive, and is associated with potentially lower morbidity and mortality than an esophagectomy. In addition, many patients who develop Barrett-associated neoplasia are elderly and may have other
medical conditions that make them poor candidates for major operative procedures like esophagogastrectomy. Current endoscopic techniques, however, may be limited in their ability to recognize the extent of dysplasia present, although newer modalities such as narrow band imaging are making endoscopic identification of dysplasia more accurate (14,15). As a result, histology remains the gold standard for dysplasia diagnosis. Endoscopic ultrasound is able to accurately identify periesophageal lymph nodes, but is less useful for determining the depth of invasion of a tumor (16,17). As a result, some investigators have advocated the use of frozen sections in evaluating EMR specimens. In a prospective study, Prasad et al. (18) examined 30 consecutive EMR cases using frozen section, and attempted to determine whether or not invasive carcinoma was present, the degree of dysplasia present, as well as the status of the deep and peripheral margins of the specimen. There was very little interobserver variation (as determined by κ scores) between the frozen section and permanent section diagnoses for both degree of dysplasia/carcinoma and margin adequacy in this study. Endoscopic ultrasound may not be accurate in determining the depth of invasion of an esophageal neoplasm, and the diagnosis of invasive adenocarcinoma on frozen section evaluation of the EMR specimen could allow the endoscopist to resect less tissue, and therefore, prevent possible procedure-related complications, since the presence of invasion is an indication for esophagectomy. In addition, the identification of a positive resection margin could allow the endoscopist to resect additional tissue to ensure complete removal of all dysplastic mucosa. However, experience is limited and it remains unclear if the additional time and expense required to perform frozen section evaluation of such EMR specimens would actually contribute significantly to patient care. In addition, frozen sections may introduce artifacts into the tissue that make their evaluation on final permanent section more difficult and endoscopy, unlike surgery, is noninvasive, and can be easily repeated in the case of a positive margin. In fact, all EMR patients with Barrett dysplasia undergo routine follow-up endoscopy regardless of the adequacy of their resections because even nondysplastic Barrett mucosa continues to be at risk for neoplastic transformation as a result of the field defect that affects the esophageal mucosa of these patients.
medical conditions that make them poor candidates for major operative procedures like esophagogastrectomy. Current endoscopic techniques, however, may be limited in their ability to recognize the extent of dysplasia present, although newer modalities such as narrow band imaging are making endoscopic identification of dysplasia more accurate (14,15). As a result, histology remains the gold standard for dysplasia diagnosis. Endoscopic ultrasound is able to accurately identify periesophageal lymph nodes, but is less useful for determining the depth of invasion of a tumor (16,17). As a result, some investigators have advocated the use of frozen sections in evaluating EMR specimens. In a prospective study, Prasad et al. (18) examined 30 consecutive EMR cases using frozen section, and attempted to determine whether or not invasive carcinoma was present, the degree of dysplasia present, as well as the status of the deep and peripheral margins of the specimen. There was very little interobserver variation (as determined by κ scores) between the frozen section and permanent section diagnoses for both degree of dysplasia/carcinoma and margin adequacy in this study. Endoscopic ultrasound may not be accurate in determining the depth of invasion of an esophageal neoplasm, and the diagnosis of invasive adenocarcinoma on frozen section evaluation of the EMR specimen could allow the endoscopist to resect less tissue, and therefore, prevent possible procedure-related complications, since the presence of invasion is an indication for esophagectomy. In addition, the identification of a positive resection margin could allow the endoscopist to resect additional tissue to ensure complete removal of all dysplastic mucosa. However, experience is limited and it remains unclear if the additional time and expense required to perform frozen section evaluation of such EMR specimens would actually contribute significantly to patient care. In addition, frozen sections may introduce artifacts into the tissue that make their evaluation on final permanent section more difficult and endoscopy, unlike surgery, is noninvasive, and can be easily repeated in the case of a positive margin. In fact, all EMR patients with Barrett dysplasia undergo routine follow-up endoscopy regardless of the adequacy of their resections because even nondysplastic Barrett mucosa continues to be at risk for neoplastic transformation as a result of the field defect that affects the esophageal mucosa of these patients.
Tumor Identification and Diagnosis
Frozen section is rarely used for the diagnosis of epithelial neoplasms of the esophagus since the majority of these are diagnosed preoperatively by endoscopic biopsy. Frozen sections, however, are sometimes requested for mesenchymal or submucosal tumors to determine their type, and to predict their behavior (benign vs. malignant) and hence, the type of operation the surgeon will perform. Unexpected mucosal lesions may also occasionally be encountered by the surgeon and may sometimes be biopsied and sent for frozen section (Fig. 12.5).
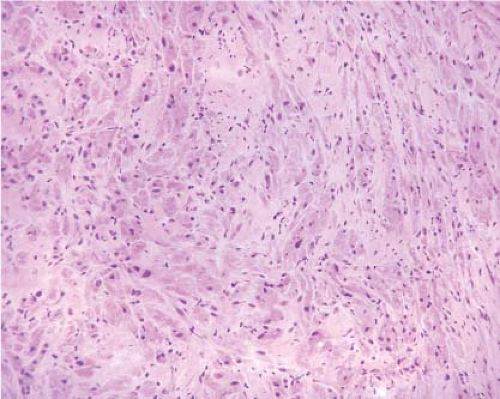
The most common mesenchymal tumor of the esophagus is a leiomyoma. Esophageal leiomyomas typically occur in adults, and often represent
incidental findings at surgery. On gross examination, leiomyomas typically appear pale pink, are firm or rubbery in consistency, and may appear lobulated. They are usually round or oval with a whorled appearance resembling their uterine counterparts. Microscopically, leiomyomas are low to moderately cellular tumors containing interlacing fascicles of bland spindle-shaped smooth muscle cells. The nuclei generally appear elongated or cigar shaped, usually without significant pleomorphism. Mitotic activity is minimal or absent.
incidental findings at surgery. On gross examination, leiomyomas typically appear pale pink, are firm or rubbery in consistency, and may appear lobulated. They are usually round or oval with a whorled appearance resembling their uterine counterparts. Microscopically, leiomyomas are low to moderately cellular tumors containing interlacing fascicles of bland spindle-shaped smooth muscle cells. The nuclei generally appear elongated or cigar shaped, usually without significant pleomorphism. Mitotic activity is minimal or absent.
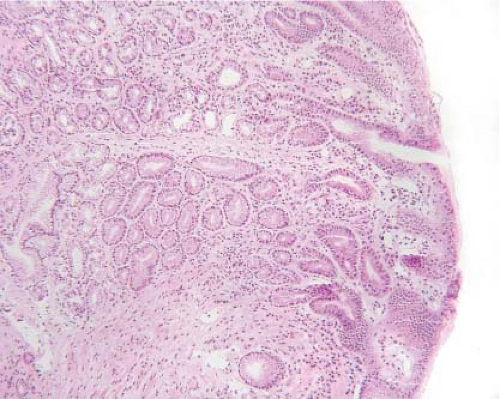 Figure 12.5 Esophageal inlet patch. A pink velvety lesion was seen by the surgeon in the proximal esophagus. An inlet patch is a benign tissue rest composed of normal-appearing gastric mucosa. |
In contrast, gastrointestinal stromal tumors (GIST) are rare in the esophagus, and most occurring in this location are malignant. Grossly, they may be intramural or polypoid, and often resemble smooth muscle tumors. They exhibit a cellular spindle cell pattern, or show areas of epithelioid differentiation. The histologic pattern varies, ranging from sheets of cells to areas with nuclear palisading and myxoid change. A definite diagnosis of GIST is often not possible on frozen section, since GISTs often resemble other spindle cell tumors. In such cases, the diagnosis of “spindle cell neoplasm, defer to permanent sections” should be made because of the significant difference in behavior of most GISTs compared with benign leiomyomas.
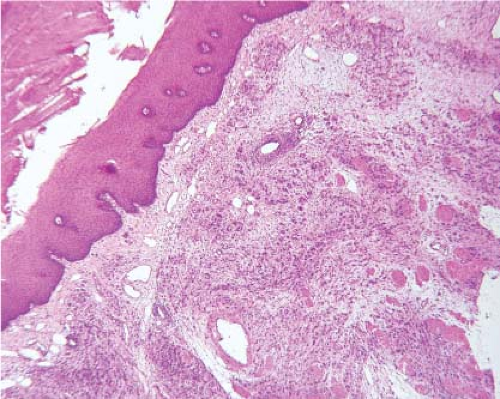
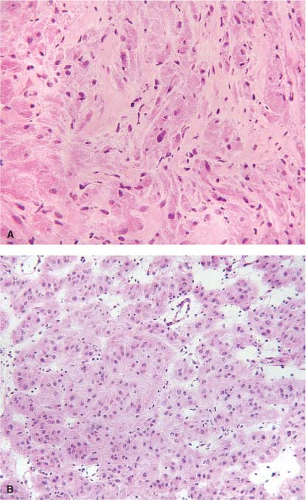
Granular cell tumors (GCT) also arise in the esophagus. Grossly, these tumors usually appear as smooth, sessile, and yellow- to grayish-white lesions. They arise in the submucosa or muscularis propria and are usually covered by a normal-appearing, intact mucosa. Esophageal GCTs
resemble GCTs arising elsewhere in the body (Fig. 12.6). Pseudoepitheliomatous hyperplasia of the overlying squamous epithelium occurs in esophageal lesions as it does in other sites. In some cases, an extensively infiltrating pattern may be seen, a feature that does not rule out a benign lesion. GCTs, however, can be malignant, although the malignant variety is rare. Features that raise the suspicion of a malignant lesion include large size (>5 cm), increased cellularity, tumor necrosis, tumor cell spindling, increased nuclear size, nuclear pleomorphism, large nucleoli, and identification of greater than two mitoses per ten high-power fields (19). GCTs may histologically resemble melanoma, carcinoma, or GIST (20) (Fig. 12.7). However, the bland cytologic features should suggest that the lesion is benign. In cases where the tumor predominantly appears to be composed of spindled cells, however, a definite distinction from GIST or other spindle cell tumors may not be possible on frozen section, and the diagnosis may need to be deferred to permanent sections. Areas of pseudoepitheliomatous hyperplasia and the clinical impression of a mass may cause granular tumors to be misinterpreted as squamous carcinomas, particularly if only a small superficial sample is examined. The epithelial cells of pseudoepitheliomatous hyperplasia generally lack cytologic atypia and the connective tissue interface is far more complicated in invasive squamous cell carcinoma than in pseudoepitheliomatous hyperplasia.
resemble GCTs arising elsewhere in the body (Fig. 12.6). Pseudoepitheliomatous hyperplasia of the overlying squamous epithelium occurs in esophageal lesions as it does in other sites. In some cases, an extensively infiltrating pattern may be seen, a feature that does not rule out a benign lesion. GCTs, however, can be malignant, although the malignant variety is rare. Features that raise the suspicion of a malignant lesion include large size (>5 cm), increased cellularity, tumor necrosis, tumor cell spindling, increased nuclear size, nuclear pleomorphism, large nucleoli, and identification of greater than two mitoses per ten high-power fields (19). GCTs may histologically resemble melanoma, carcinoma, or GIST (20) (Fig. 12.7). However, the bland cytologic features should suggest that the lesion is benign. In cases where the tumor predominantly appears to be composed of spindled cells, however, a definite distinction from GIST or other spindle cell tumors may not be possible on frozen section, and the diagnosis may need to be deferred to permanent sections. Areas of pseudoepitheliomatous hyperplasia and the clinical impression of a mass may cause granular tumors to be misinterpreted as squamous carcinomas, particularly if only a small superficial sample is examined. The epithelial cells of pseudoepitheliomatous hyperplasia generally lack cytologic atypia and the connective tissue interface is far more complicated in invasive squamous cell carcinoma than in pseudoepitheliomatous hyperplasia.
Stomach: Major Intraoperative Questions
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree