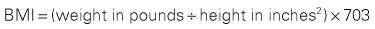Denise Jackson
Gastrointestinal Surgery
Surgeons embark on surgery of the gastrointestinal (GI) system to diagnose, remove, or prevent the progression of pathologic conditions. When cure is not possible, palliative measures to relieve symptoms can provide comfort and nutrition, and promote quality of life and dignity in death. Advances in technology and the science of human digestion, metabolism, immunology, and inflammatory responses to illness and injury have greatly broadened the spectrum of possible modalities and approaches to manage and treat patients with GI disorders. Clinical research uses patient outcomes to compare the effectiveness of new approaches and innovative methods to more traditional methods of GI procedures; that research continues to grow rapidly, providing new evidence for practice and decision making (Anderson et al, 2012; Cone et al, 2011; DeMaria et al, 2011; Fried, 2012; Mason, 2012).
GI surgery comprises the surgical management of pathologic conditions of the esophagus, stomach, and small and large intestines. Gastroenterology is a medical specialty that uses endoscopy to locate, identify, mark, and sometimes treat intraluminal pathology in the GI tract. Endoscopic procedures are a critical component of surgical diagnosis, staging of disease, and postoperative evaluation. Although general surgeons and surgical teams may specialize in laparoscopy, surgical endoscopy, bariatrics, surgical oncology, or colorectal surgery, perioperative nurses and scrub personnel must be familiar with the fundamentals of GI surgery and common patient care requirements.
Surgical Anatomy
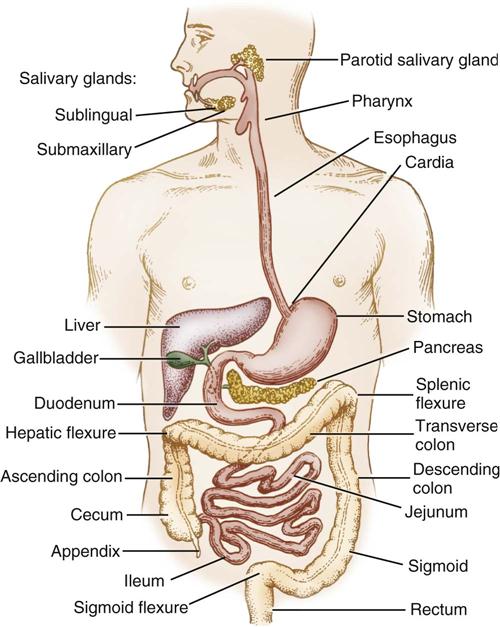
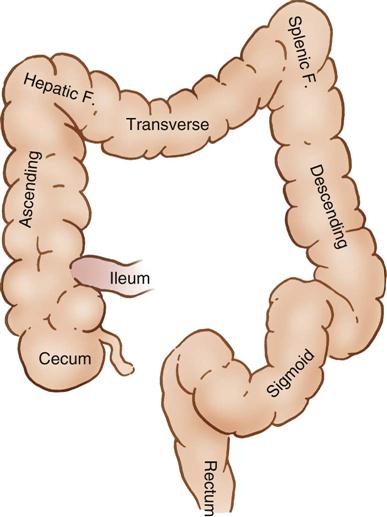
The GI tract, or alimentary canal, is a continuous, tubelike structure that spans the human torso (Figure 11-1). It includes the mouth; pharynx; esophagus; stomach; small intestine, consisting of the duodenum, jejunum, and ileum; and large intestine. The large intestine consists of the cecum, ascending colon, transverse colon, descending colon, sigmoid colon, rectum, and anus. The length of the GI tract is about 6 meters (20 feet). Basic functions of the GI tract are ingestion, secretion, mixing, digestion, propulsion, absorption, and elimination by defecation. The GI tract is a complex microbiologic ecosystem that supports and maintains essential digestive and protective functions vital to life. Substantial populations of microorganisms—both obligate anaerobes and facultative bacterial spores—exist in the intestinal lumen. The organisms of the upper tract differ from those of the lower tract, with the highest concentration in the distal bowel. These organisms can contribute to contamination and disease processes within the intestinal tract and throughout the body.
The esophagus is a collapsible musculomembranous tube through which ingested material moves, by peristalsis, from the pharynx to the stomach. After exiting the pharynx, the esophagus passes through the neck, posterior to the trachea and anterior to the vertebral column. The mediastinal portion of the esophagus enters the thoracic cavity at the level of the first thoracic vertebra where it lies posterior to the heart. The esophagus enters the peritoneal cavity through the esophageal hiatus of the diaphragm, where it joins the cardia of the stomach. The lower esophageal sphincter (LES) is at the terminal end of the esophagus; its function is to prevent reflux of gastric contents. Blood supply to the esophagus originates from branches of the inferior thyroid arteries, bronchial arteries, the thoracic aorta, and branches of the left gastric and inferior phrenic arteries. The venous drainage from the esophagus empties into the subclavian veins, the azygos vein on the right, the hemiazygos vein on the left, and through the coronary vein in the portal circulation. The nerve supply is from branches of the vagus nerve and the sympathetic nervous system.
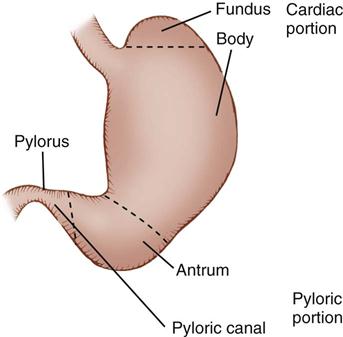
The stomach lies in the upper abdominal cavity, to the left of the midline, inferior to the diaphragm and anterior to the pancreas. It is attached to the spleen on the left by the gastrosplenic ligament, the liver on the right by the hepatogastric ligament, the duodenum on the right by the gastroduodenal ligament, and the transverse colon inferiorly by the posterior layer of the greater omentum. Physiologically cells in different regions of the stomach have unique functions related to digestion. Description of the stomach is by names related to each region, such as the cardia, fundus, body, antrum, and pyloric canal (Figure 11-2). The fundus lies inferior to the left lobe of the liver and medially adjacent to the spleen. Inferior to the fundus the body and antrum curve obliquely to the right, before terminating at the pylorus. The concave, or medial, margin of the stomach is referred to as the lesser curve and the convex, or lateral, margin of the stomach is known as the greater curve.
The omentum is a double layer of fatty peritoneum attached along the greater curve of the stomach. It drapes down loosely over the intestines and then folds posteriorly on itself before sweeping upward to attach along the transverse colon. The mesentery is also a double layer of peritoneum, but it differs from the omentum in that it connects the small bowel and parts of the colon to the posterior abdominal wall and contains the blood supply, nerves, and lymphatics of the intestines within its layers.
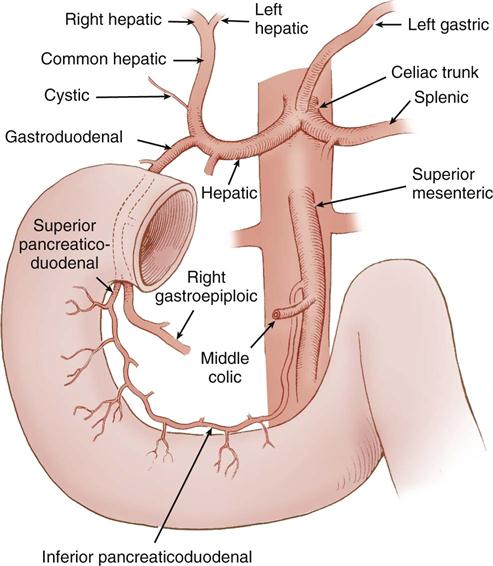
The blood supply to the stomach originates at the celiac trunk of the aorta, which branches into the left gastric, splenic, and common hepatic arteries. The right gastric and gastroduodenal arteries are branches of the common hepatic artery. The left and right gastric arteries supply the lesser curve of the stomach; the stomach’s greater curve receives its supply from the right gastroepiploic artery, a branch of the gastroduodenal artery, and the left gastroepiploic artery, which originates from the splenic artery. Additional blood supply comes from the inferior phrenic arteries and the short gastric arteries located between the greater curve and the spleen. The gastric veins follow the same pathways as the arteries and drain into the portal vein. The right gastroepiploic vein drains into the superior mesenteric vein and the left gastroepiploic vein drains into the splenic vein. Regional lymph nodes are located at the gastroesophageal junction, the splenic hilum, and the pylorus; they receive gastric lymphatic drainage, all of which empties into the celiac nodes and ultimately into the cisterna chyli and thoracic duct. The autonomic, sympathetic, parasympathetic, and enteric nervous systems regulate gastric function.
The stomach receives ingested food from the esophagus to begin the digestive process; it then propels partially digested food, or chyme, into the duodenum through the pylorus. In anticipation of receiving ingested material, the stomach prepares for its role in digestion by increasing motility, producing gastric acid, and releasing pepsinogen, intrinsic factor, gastric fluids, and mucus. Peptide hormones in the stomach (gastrin, somatostatin, and ghrelin) function directly and indirectly to regulate gastric secretions and motility. Gastrin causes enterochromaffin-like (ECL) cells in the body of the stomach to release histamine, thereby stimulating gastric acid secretion from the parietal cells. Somatostatin inhibits gastrin, histamine, and acid secretion. Production of ghrelin, a peptide hormone discovered in 1999, occurs in the fundus. It stimulates gastric motility as well as the appetite, regulates hunger and satiety during meals, and plays a role in long-term body weight regulation (King and Hines, 2013). Research regarding ghrelin’s role in obesity and potential medications for the treatment of obesity is ongoing (Mahvi and Krantz, 2010).
The small intestine is the longest part of the digestive tract. It begins at the pylorus and ends at the ileocecal valve. The adult small intestine varies in length but is usually about 3 meters long with a diameter of approximately 2.5 cm. It has three parts: the duodenum, the jejunum and the ileum. The length of the duodenum is about 20 cm; the jejunum, approximately 110 cm; and the ileum, on the order of 155 cm long.
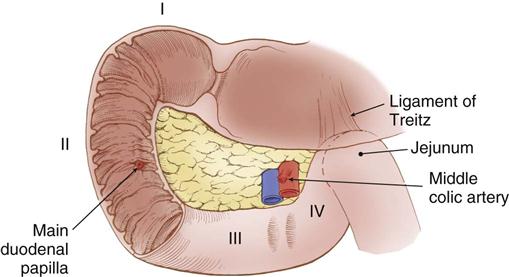
The first portion of the small intestine is the duodenum. Its proximal 2 cm are mobile and considered to be intraperitoneal, whereas the remaining duodenum is retroperitoneal and fixed posteriorly by peritoneal attachments. The first part of the duodenum, called the duodenal bulb, dilates. Characteristic circular folds of the small intestinal mucosa begin in the duodenum, continue through the jejunum, and become less prominent in the ileum. The purpose of these folds, called plicae circulares of Kerckring, is to provide greater mucosal surface area for absorption of nutrients. The duodenum forms a C-shaped curve around the head of the pancreas. The common bile duct and the main pancreatic duct enter the posteromedial wall of the duodenum at the ampulla of Vater (also referred to as the main duodenal papilla) within this curve (Figure 11-3). The duodenum continues in a medial direction along the inferior border of the body of the pancreas before it ascends slightly to the duodenojejunal flexure, at which point it becomes the jejunum.
The blood supply to the duodenum comes from the gastroduodenal artery, located behind the duodenal bulb (Figure 11-4). At the inferior margin of the bulb, the gastroduodenal artery divides into the right gastroepiploic and superior pancreaticoduodenal arteries to supply the proximal duodenum and head of the pancreas. The inferior pancreaticoduodenal artery, a branch of the superior mesenteric artery, supplies the transverse and ascending portions of the duodenum, as well as the head and body of the pancreas.
In the left upper quadrant, the ligament of Treitz supports the duodenojejunal flexure and serves as a landmark to identify the beginning of the jejunum. The jejunum continues for about 2.5 meters (8 feet) before it transitions into the ileum in the right lower quadrant. The ileum continues for about another 3.5 meters (12 feet) before it ends at the ileocecal junction.
The blood supply to the jejunum and ileum comes entirely from their respective branches of the superior mesenteric artery. The mesentery of the jejunum and ileum is about 5.5 meters (18 feet) long, accounting for its mobility within the abdominal cavity. Along its antimesenteric, or opposite, border, the mucosa of the ileum contains major deposits of lymphatic tissue, known as Peyer patches. The rich lymphatic drainage of the small bowel plays a major role in fat absorption. Lymphatic drainage from the mucosa proceeds through the wall of the small intestine to lymph nodes adjacent to the mesentery. It then proceeds to larger lymphatics that communicate with the retroperitoneal cisterna chyli and from there to the thoracic duct, where it terminates in the internal jugular vein. The lymphatics of the intestine play a major role, both in the body’s immune defense and in the distribution of cells arising from intestinal neoplasms.
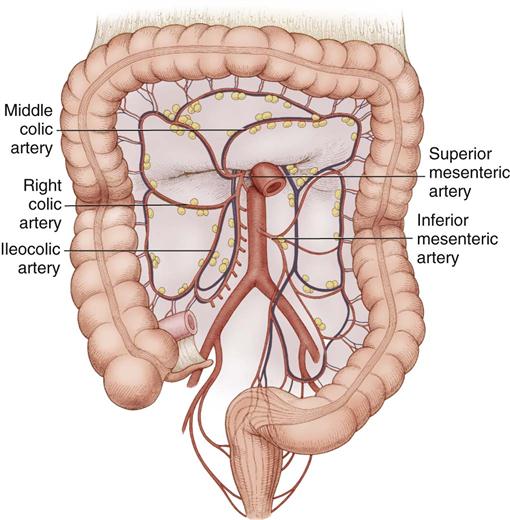
The large intestine (Figure 11-5) consists of the cecum, colon, rectum, and anal canal (not illustrated). The cecum attaches to the lateral abdominal wall by peritoneal attachments called cecal folds. The terminal branch of the superior mesenteric artery, the ileocolic artery, supplies blood to the cecum.
The appendix is a 7- to 10-cm wormlike structure that arises from the posteromedial wall of the cecum, below the ileocecal junction, and attaches to the cecum by the mesoappendix. The blood supply to the appendix comes from the appendicular artery, which branches from the terminal part of the ileocolic artery.
The ascending colon extends upward from the cecum to the hepatic flexure, where it lies behind the right lobe of the liver and in front of the anterior surface of the right kidney. It receives its blood supply from the ileocolic and middle colic arteries (Figure 11-6). The transverse colon begins at the hepatic flexure and ends at the splenic flexure. It lies below the stomach, suspended by the transverse mesocolon. The blood supply to the transverse colon is via the middle colic artery (see Figure 11-6). The descending colon extends downward from the splenic flexure to become the sigmoid colon. The sigmoid colon makes an S-shaped curve toward the midline, where it descends to transition into the rectum at the rectosigmoid junction. The arterial supply to the descending and sigmoid colon comes from the left colic and sigmoid arteries. The superior, middle, and inferior rectal arteries supply the rectum and anal canal. The marginal artery of Drummond forms an arcade of collateral circulation along the inner border of the colon.
Distinguishing physical characteristics of the colon are the teniae coli, epiploic appendices, and haustra. The teniae coli are three separate, longitudinal bands of muscle distributed along the length of the colon. The epiploic appendices are fatty appendages attached to the colon along the teniae and have no particular function. Haustra are sacculations of the colon wall along the contracted teniae, giving it a segmented appearance.
The rectum begins at the rectosigmoid junction, where the teniae coli merge at the level of the sacral promontory. The absence of distinct teniae serves as a visual landmark for the upper margin of the rectum. The rectum has three characteristic lateral curves, or flexures, that coincide internally with transverse rectal folds called valves of Houston (Fry et al, 2010). The rectum lies on the sacrum posteriorly. As it descends into the pelvis, the posterior rectum attaches to the anterior surface of the sacrum by the rectosacral fascia. It also attaches to the pelvis by fascia extending from the right and left pelvic sidewalls. In the male, the rectovesical fascia, also called Denonvilliers fascia, separates the anterior rectum from the seminal glands, prostate, bladder, ureters, and ductus deferens. In the female the rectovaginal fascia separates the anterior rectum from the vagina. The terminal rectum, or ampulla, dilates to receive and hold feces prior to passage into the anal canal. The anal canal is the most distal portion of the GI tract. It is a 3- to 5-cm funnel-like passage that begins at the anorectal junction, lies between the internal and external anal sphincters, and ends at the anal verge.
The large intestine recovers water, electrolytes, and nutrients from succus entericus, a mixture of undigested fluids, starches, proteins, and bile salts received from the ileum. The remaining waste product descends toward the rectum, solidifies into feces, and stores in the rectal ampulla until its expulsion through the anal canal, or, commonly, defecation.
Perioperative Nursing Considerations
GI surgery and endoscopic procedures vary widely, depending on the patient’s medical diagnosis, the purpose of the intended procedure, the surgical approach, and the involved anatomic structures. Other influencing factors include the patient’s goals and desire for treatment, the surgeon’s preferred approach based on training and experience, and the availability of advanced technologies in imaging, access, and instrumentation.
Assessment
Patient-centered care begins with an assessment that integrates information about the physiologic and psychosocial status of the patient into an individualized plan of care. Key sources of information that facilitate and guide the assessment process include medical history, physical examination, physician orders, and results from laboratory and other diagnostic studies. Laboratory studies might include a complete blood count, as well as a complete metabolic panel to measure serum glucose, serum electrolytes, kidney function, liver function, and serum proteins. Coagulation studies such as international normalized ratio (INR) and prothrombin time or partial thromboplastin time (PT/PTT) may be appropriate if indicated (Mehrdad and Marks, 2011; Stonemetz, 2011). Abnormal values require specific notation and communication to the surgeon and anesthesia provider (lab values are presented in Appendix A).
Diagnostic procedures, determined by the patient’s presenting signs and symptoms, the location of suspected pathology, and the potential sites of metastasis may include esophageal manometry, pH monitoring, and motility studies. Additional tests may include radiologic studies, with or without contrast markers, endoscopic examinations, abdominal or endoscopic ultrasound (EUS), computed tomography (CT) imaging scans, magnetic resonance imaging (MRI), or positron emission tomography (PET). Technologic advances in endoscopic imaging include EUS, chromoendoscopy, confocal laser endomicroscopy, endocystoscopy, optical coherence tomography (Friedland and Van Dam, 2012), magnification endoscopy (Guelrud, 2012), narrow band imaging (Bergman, 2012), and wireless video capsule endoscopy (Cave, 2012). These provide enhanced details of GI anatomy and are essential tools in identifying and staging GI malignancies in order to determine surgical options and other treatments.
Barriers to patient communication such as language, sensory deficits, altered cognitive function, and distractors, such as pain or emotional stress, require assessment throughout the preoperative interview. The perioperative nurse needs to acknowledge tearfulness, as well as expressions of fear, anger, denial, depression, nervousness, and anxiety, as legitimate, and to encourage the patient to verbalize his or her concerns.
After confirming the patient’s identity, the perioperative nurse asks the patient to verify, in his or her own words, an understanding of the scheduled procedure, the planned approach, and laterality if applicable (Patient-Centered Care). Due to the nature and unpredictability of GI surgery, if a minimally invasive approach is planned, the patient must understand the possibility of the need to convert to a laparotomy. If intestinal diversion (ileostomy, colostomy) is a possibility, the perioperative nurse determines if a wound ostomy continence nurse (WOCN) has marked the site of the stoma and assesses the patient’s understanding and acceptance of this part of the procedure. Individualized ostomy site selection by a WOCN helps prevent appliance leaks and peristomal skin complications (Maidl and Ohland, 2012). Optimally, the site of the ostomy is within the rectus muscle to decrease risk of peristomal hernias; is not placed in or across a skinfold, crease, or at the belt line; and is visible and easily accessible to enable the patient to perform self-care postoperatively. A WOCN’s preoperative counseling and education, aimed at assisting the patient and family to adapt to expected lifestyle changes, to accept altered body image, to address sexuality concerns, and to understand elements of ostomy self-care, have demonstrated a positive effect on long-term outcomes (Doughty and Landmann, 2012).
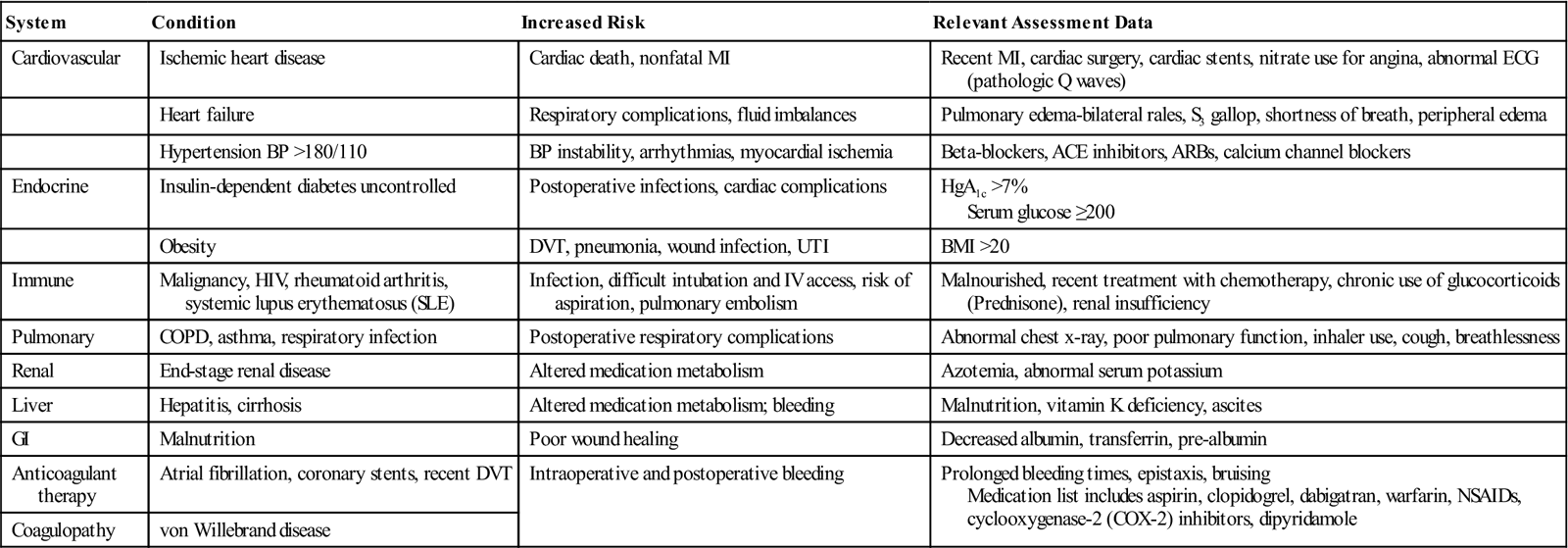
The perioperative nurse checks the review of systems (Table 11-1) in the medical record to identify and confirm patient conditions that can increase the risks of perioperative complications.
TABLE 11-1
Preoperative Nursing Review of Systems
| System | Condition | Increased Risk | Relevant Assessment Data |
| Cardiovascular | Ischemic heart disease | Cardiac death, nonfatal MI | Recent MI, cardiac surgery, cardiac stents, nitrate use for angina, abnormal ECG (pathologic Q waves) |
| Heart failure | Respiratory complications, fluid imbalances | Pulmonary edema-bilateral rales, S3 gallop, shortness of breath, peripheral edema | |
| Hypertension BP >180/110 | BP instability, arrhythmias, myocardial ischemia | Beta-blockers, ACE inhibitors, ARBs, calcium channel blockers | |
| Endocrine | Insulin-dependent diabetes uncontrolled | Postoperative infections, cardiac complications | HgA1c >7% Serum glucose ≥200 |
| Obesity | DVT, pneumonia, wound infection, UTI | BMI >20 | |
| Immune | Malignancy, HIV, rheumatoid arthritis, systemic lupus erythematosus (SLE) | Infection, difficult intubation and IV access, risk of aspiration, pulmonary embolism | Malnourished, recent treatment with chemotherapy, chronic use of glucocorticoids (Prednisone), renal insufficiency |
| Pulmonary | COPD, asthma, respiratory infection | Postoperative respiratory complications | Abnormal chest x-ray, poor pulmonary function, inhaler use, cough, breathlessness |
| Renal | End-stage renal disease | Altered medication metabolism | Azotemia, abnormal serum potassium |
| Liver | Hepatitis, cirrhosis | Altered medication metabolism; bleeding | Malnutrition, vitamin K deficiency, ascites |
| GI | Malnutrition | Poor wound healing | Decreased albumin, transferrin, pre-albumin |
| Anticoagulant therapy | Atrial fibrillation, coronary stents, recent DVT | Intraoperative and postoperative bleeding | Prolonged bleeding times, epistaxis, bruising Medication list includes aspirin, clopidogrel, dabigatran, warfarin, NSAIDs, cyclooxygenase-2 (COX-2) inhibitors, dipyridamole |
| Coagulopathy | von Willebrand disease |
Modified from Hassilgren P et al: Perioperative management: practical principles, molecular basis of risk and future direction. In Fischer J, editor: Fischer’s mastery of surgery, Philadelphia, 2012, Lippincott Williams & Wilkins.
Current medications, including use of supplements, require review with the patient. Verify the last dose taken on all medications. Conditions of the GI tract requiring surgical intervention are often accompanied by alterations in nutritional status, which can cause increased risk for infection, poor wound healing, incidence of pressure ulcers, and overgrowth of bacteria in the GI tract (Tess and Fairfield, 2012). Assessment of the patient’s nutritional status includes determining the patient’s body mass index (BMI) (Box 11-1), history of recent weight loss or gain, difficulty swallowing, nausea, vomiting, diarrhea, constipation, and NPO status.
Components of a nursing physical assessment include notation of general appearance and body-mass distribution. Assessments of skin integrity, quality of peripheral pulses, presence of neuropathies, and physical limitations help determine baseline data for evaluation of outcomes related to positioning and hydration status. Focused examination of the patient’s abdomen includes inspection for skin integrity, hair distribution, scars, and distention. Palpation to assess for rigidity, referral and/or localization of pain, masses, and unusual pulsations may follow if indicated.
Relevant social assessment includes use of tobacco, alcohol, or recreational drugs. Inquiries about the patient’s ability to perform activities of daily living, circumstances of the patient’s home situation, and availability of family, friends, or other support systems during hospitalization and after discharge play an important part of discharge planning.
Nursing Diagnosis
Nursing diagnoses related to the care of patients undergoing GI surgery might include the following:
• Anxiety related to perioperative events
• Deficient Knowledge related to impending surgery
• Disturbed Body Image related to intestinal diversion (when diversion possible or planned)
• Risk for Imbalanced Body Temperature
• Risk for Infection at the surgical site
• Risk for Perioperative Positioning Injury
Outcome Identification
Outcomes identified for selected nursing diagnoses could be stated as follows:
Planning
The preoperative assessment provides the perioperative nurse with critical information to develop an individualized plan of care. Analysis of patient-specific physiologic and psychosocial data assist in identifying nursing interventions to comfort patients, mitigate risks for complications, prevent injury and infection, and help the patient to achieve the best possible outcomes. Planning for optimal patient care also includes ensuring that necessary instruments, supplies, and equipment are at hand for the planned procedure. A Sample Plan of Care for patients undergoing GI surgery can be found on p. 301.
Implementation
The perioperative nurse implements the plan of care throughout all phases of the patient’s surgical experience. Communication and cooperation between all members of the surgical team, working together to help the patient achieve identified outcomes, is essential. There is strong evidence that preoperative checklists prevent a number of surgical safety events (Shekelle et al, 2013).
Most patients undergoing open and laparoscopic GI surgery require general endotracheal anesthesia. A midthoracic epidural, placed preoperatively as an adjuvant to general anesthesia, can decrease postoperative pain, increase adherence to pulmonary exercises, and promote early ambulation. Intravenous (IV) moderate sedation is appropriate for outpatient endoscopic procedures, such as esophagogastroduodenoscopy (EGD) or colonoscopy. Monitoring devices and parameters typical to general and epidural anesthesia techniques appear in Chapter 5.
Fluid management during significant blood loss may require arterial monitoring and frequent intraoperative sampling of hemoglobin and hematocrit (H&H), arterial blood gases (ABGs), electrolytes, and coagulation studies. Preoperatively the physician may order a type and screen or type and crossmatch in anticipation of intraoperative blood/blood component transfusion. Replacement fluids include packed red blood cells (PRBCs), albumin, platelets, fresh frozen plasma, electrolytes, and colloids or crystalloids.
Prior to elective surgery, patients may choose to donate 1 or 2 units of autologous blood for use at surgery. Friends and family members may also donate donor-directed, compatible blood. Autotransfusion, or cell salvage, of the patient’s blood during surgery may not be appropriate, given potential contamination from bowel contents or from malignant GI tumors. A nasogastric (NG) tube, inserted to decompress the stomach, suctions gastric secretions as well. Insertion of a urinary catheter decompresses the bladder and provides accurate measurement of urinary output and renal function.
Administration of IV antibiotics occurs before the incision and repeats as needed to maintain adequate levels throughout the procedure (Surgical Pharmacology). Antibiotic solutions may be used for irrigation during the procedure and before closure. Additional intraoperative medications may be appropriate per institutional standard or surgeon preference. Use of hemostatic agents, anticoagulants, steroid preparations, and local anesthetics is possible also. The best guide for planning medications, therefore, is a comprehensive and frequently updated pick-list, or surgeon/procedure preference sheet.
Surgical Pharmacology
Commonly Administered Antibiotics in GI Surgery
| Medication/Category | Dosage/Route | Purpose/Action | Adverse Reactions | Nursing Implications |
| Cefazolin (first-generation cephalosporin) | <80 kg: IV 1 g 81-160 kg: IV 2 g >160 kg: IV 3 g | Enteric gram-negative bacilli, gram-positive cocci | Administer within 1 hr of incision | |
| Cefoxitin (second-generation cephalosporin) | IV 1-2 g | Enteric gram-negative bacilli, enterococci, clostridia | Administer within 1 hour of incision | |
| Ampicillin sulbactam (aminopenicillin and beta-lactamase inhibitor) | IV 1.5-3 g q6hr | Enterococcus faecalis, gram-negative bacilli | Diarrhea, nausea | Administer within 1 hr of incision |
| Clindamycin | <80 kg: IV 600 mg 81-160 kg: IV 900 mg >160 kg: IV 1200 mg | Aerobes, anaerobes, enteric gram-negative bacilli | Nausea | Dilute each 300 mg in 50 mL D5W and give over 10-60 min |
| Ertapenem/carbapenem | IV 1 g | Enterobacter, Escherichia coli | Adjust dose for renal impairment | |
| Metronidazole hydrochloride | <80 kg: IV 500 mg 81-160 kg: IV 1000 mg >160 kg: IV 1500 mg | Anaerobes | Headache, nausea, vaginitis, candida | Infuse over 1 hr Do not give IV push Incompatible with aztreonam and ceftriaxone |
| Vancomycin (glycopeptide) | IV 1000 mg or 10-15 mg/kg over 1 hr | MRSA, Staphylococcus aureus, Enterococcus | Erythematous rash on face and upper body, hypotension | Infuse over 1 hr |
| Ciprofloxacin (fluoroquinolone) | IV 400 mg | Gram-negative bacilli, Streptococcus pneumoniae, gram-positive cocci, mycobacterial species | Rash, anorexia, nausea, vomiting, headache, dizziness, phototoxicity | Administer within 1 hr of incision |
| Levofloxacin (fluoroquinolone) | IV 750 mg | Gram-negative bacilli, S. pneumoniae, gram-positive cocci, mycobacterial species | Administer within 1 hr of incision | |
| Aztreonam (miscellaneous) | IV 1-2 g | Gram-negative bacteria | Administer within 1 hr of incision | |
| Gentamicin | IV 1-2.5 mg/kg | Gram-negative bacteria | Administer within 1 hr of incision |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree