OBJECTIVES
After studying this chapter, you should be able to:
Name the key hormones secreted by Leydig cells and Sertoli cells of the testes.
Outline the steps involved in spermatogenesis.
Outline the mechanisms that produce erection and ejaculation.
Know the general structure of testosterone, and describe its biosynthesis, transport, metabolism, and actions.
Describe the processes involved in regulation of testosterone secretion.
INTRODUCTION
The role for a functional, secreting testis in the formation of male genitalia, the action of male hormones on the brain in early development, and development of the male reproductive system through adolescence and into adulthood were discussed in the previous chapter. As observed in the female, male gonads have a dual function: the production of germ cells (gametogenesis) and the secretion of sex hormones. The androgens are the steroid sex hormones that are masculinizing in their action. The testes secrete large amounts of androgens, principally testosterone, but they also secrete small amounts of estrogens. Unlike that observed in females, male gonadotropin secretion is noncyclical, and once mature, male gonadal function slowly declines with advancing age, but the ability to produce viable gametes persists. In this chapter, the discussion will be focused on the structure and physiology of the mature male reproductive system.
THE MALE REPRODUCTIVE SYSTEM
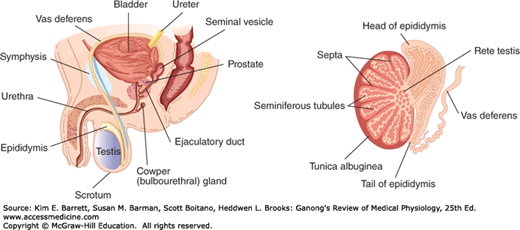
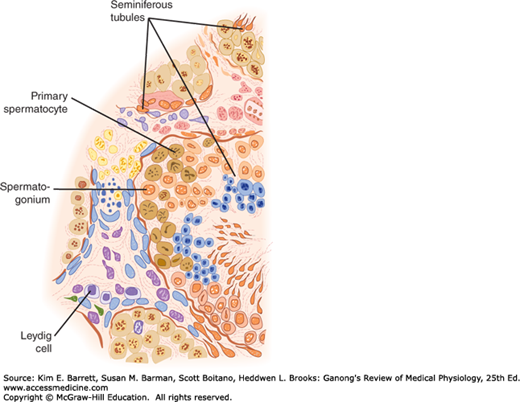
The testes are made up of loops of convoluted seminiferous tubules, in the walls of which the spermatozoa are formed from the primitive germ cells (spermatogenesis). Both ends of each loop drain into a network of ducts in the head of the epididymis. From there, spermatozoa pass through the tail of the epididymis into the vas deferens. They enter through the ejaculatory ducts into the urethra in the body of the prostate at the time of ejaculation (Figure 23–1). Between the tubules in the testes are nests of cells containing lipid granules, the interstitial cells of Leydig (Leydig cells; Figures 23–2 and 23–3), which secrete testosterone into the bloodstream. The spermatic arteries to the testes are tortuous, and blood in them runs parallel but in the opposite direction to blood in the pampiniform plexus of spermatic veins. This anatomic arrangement may permit countercurrent exchange of heat and testosterone. The principles of countercurrent exchange are considered in detail in relation to the kidney in Chapter 37.
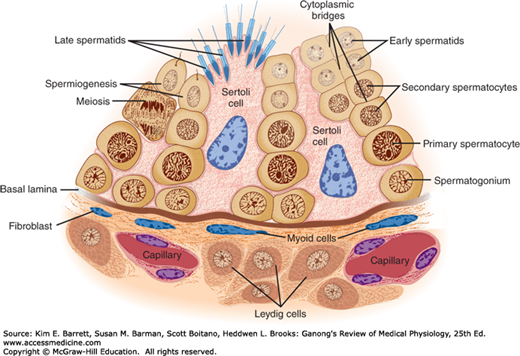
FIGURE 23–3
Seminiferous epithelium. Note that maturing germ cells remain connected by cytoplasmic bridges through the early spermatid stage and that these cells are closely invested by Sertoli cell cytoplasm as they move from the basal lamina to the lumen. (Reproduced with permission from Junqueira LC, Carneiro J: Basic Histology: Text & Atlas, 10th ed. New York, NY: McGraw-Hill; 2003.)
The walls of the seminiferous tubules are lined by primitive germ cells and Sertoli cells, large, complex glycogen-containing cells that stretch from the basal lamina of the tubule to the lumen (Figure 23–3). Germ cells must stay in contact with Sertoli cells to survive; this contact is maintained by cytoplasmic bridges. Tight junctions between adjacent Sertoli cells near the basal lamina form a blood-testis barrier that prevents many large molecules from passing from the interstitial tissue and the part of the tubule near the basal lamina (basal compartment) to the region near the tubular lumen (adluminal compartment) and the lumen. However, steroids penetrate this barrier with ease, and evidence suggests that some proteins also pass from the Sertoli cells to the Leydig cells, and vice versa, to function in a paracrine manner. In addition, maturing germ cells must pass through the barrier as they move to the lumen. This appears to occur without disruption of the barrier by coordinated breakdown of the tight junctions above the germ cells and formation of new tight junctions below them.
The fluid in the lumen of the seminiferous tubules is quite different from plasma; it contains very little protein and glucose but is rich in androgens, estrogens, K+, inositol, and glutamic and aspartic acids. Maintenance of its composition depends on the blood-testis barrier. The barrier also protects the germ cells from bloodborne noxious agents, prevents antigenic products of germ cell division and maturation from entering the circulation and generating an autoimmune response, and may help establish an osmotic gradient that facilitates movement of fluid into the tubular lumen.
Spermatogonia, the primitive germ cells next to the basal lamina of the seminiferous tubules, mature into primary spermatocytes (Figure 23–3). This process begins during adolescence. The primary spermatocytes undergo meiotic division, reducing the number of chromosomes. In this two-stage process, they divide into secondary spermatocytes and then into spermatids, which contain the haploid number of 23 chromosomes. The spermatids mature into spermatozoa (sperm). As a single spermatogonium divides and matures, its descendants remain tied together by cytoplasmic bridges until the late spermatid stage. This arrangement helps ensure synchrony of the differentiation of each clone of germ cells. The estimated number of spermatids formed from a single spermatogonium is 512. The formation of a mature sperm from a primitive germ cell by spermatogenesis in humans spans approximately 74 days.
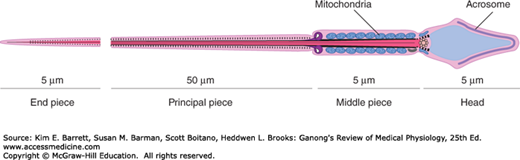
Each sperm is an intricate motile cell, rich in DNA, with a head that is made up mostly of chromosomal material (Figure 23–4). Covering the head like a cap is the acrosome, a lysosome-like organelle rich in enzymes involved in sperm penetration of the ovum and other events associated with fertilization. The motile tail of the sperm is wrapped in its proximal portion by a sheath holding numerous mitochondria. The membranes of late spermatids and spermatozoa contain a special small form of angiotensin-converting enzyme called germinal angiotensin-converting enzyme (gACE). Germinal ACE is transcribed from the same gene as the somatic ACE (sACE); however, gACE displays tissue-specific expression based on alternative transcription initiation sites and alternate splicing patterns. The full function of gACE has yet to be elucidated, although gACE-specific knockout mouse models are sterile.
Spermatids mature into spermatozoa in deep folds of the cytoplasm of the Sertoli cells (Figure 23–3). Mature spermatozoa are released from the Sertoli cells and become free in the lumens of the tubules. The Sertoli cells secrete androgen-binding protein (ABP), inhibin, and MIS. They do not synthesize androgens, but they contain aromatase (CYP19), the enzyme responsible for conversion of androgens to estrogens, and they can produce estrogens. ABP probably functions to maintain a high, stable supply of androgen in the tubular fluid. Inhibin inhibits follicle-stimulating hormone (FSH) secretion.
FSH and androgens maintain the gametogenic function of the testis. After hypophysectomy, injection of luteinizing hormone (LH) produces a high local concentration of androgen in the testes, and this maintains spermatogenesis. The stages from spermatogonia to spermatids appear to be androgen-independent. However, the maturation from spermatids to spermatozoa depends on androgen acting on the Sertoli cells in which the developing spermatozoa are embedded. FSH acts on the Sertoli cells to facilitate the last stages of spermatid maturation. In addition, it promotes the production of ABP.
An interesting observation is that the estrogen content of the fluid in the rete testis (Figure 23–1) is high, and the walls of the rete testis contain numerous α estrogen receptors (ERα). In this region, fluid is reabsorbed and the spermatozoa are concentrated. If this does not occur, the sperm entering the epididymis are diluted in a large volume of fluid, resulting in reduced fertility.
Spermatozoa leaving the testes are not fully mobile. They continue their maturation and acquire motility during their passage through the epididymis. Motility is obviously important in vivo, but fertilization occurs in vitro if an immotile spermatozoon from the head of the epididymis is microinjected directly into an ovum. The ability to move forward (progressive motility), which is acquired in the epididymis, involves activation of a unique set of proteins from the CatSper family, which are localized to the principal piece of the sperm tail. CatSpers form an alkaline-sensitive Ca2+ channel that becomes more active as the sperm go from the acidic vagina (pH ~5) to the cervical mucus (pH ~8). Sperm from knockout mice that do not express CatSper1-4 have altered motility and are infertile, emphasizing the important role of these proteins. In addition, spermatozoa express olfactory receptors, and ovaries produce odorant-like molecules. Recent evidence indicates that these molecules and their receptors interact, fostering movement of the spermatozoa toward the ovary (chemotaxis).
Ejaculation of the spermatozoon involves contractions of the vas deferens mediated in part by P2X receptors, ligand-gated cation channels that respond to ATP (see Chapter 7), and fertility is reduced in mice in which these receptors are knocked out.
Once ejaculated into the female, the spermatozoa move up the uterus to the isthmus of the uterine tubes, where they slow down and undergo capacitation. This further maturation process involves two components: increasing the motility of the spermatozoa and facilitating their preparation for the acrosome reaction. However, the role of capacitation appears to be facilitatory rather than obligatory, because fertilization is readily produced in vitro. From the isthmuses, the capacitated spermatozoa move rapidly to the tubal ampullas, where fertilization takes place.
Spermatogenesis requires a temperature considerably lower than that of the interior of the body. The testes are normally maintained at a temperature of about 32°C. They are kept cool by air circulating around the scrotum and probably by heat exchange in a countercurrent manner between the spermatic arteries and veins. When the testes are retained in the abdomen or when, in experimental animals, they are held close to the body, degeneration of the tubular walls and sterility result. Situations that increase heat around the testes in humans (eg, hot baths [43–45°C for 30 min/d] and insulated athletic supporters) can reduce sperm counts, in some cases by 90%. However, the reductions produced in this manner are not consistent enough to make the procedures reliable forms of male contraception. In addition, evidence suggests a seasonal effect in men, with sperm counts being greater in the winter regardless of the temperature to which the scrotum is exposed.
The fluid that is ejaculated at the time of orgasm, the semen, contains sperm and the secretions of the seminal vesicles, prostate, Cowper glands, and, probably, the urethral glands (Table 23–1). An average volume per ejaculate is 2.5–3.5 mL after several days of abstinence from sexual activity. The volume of semen and the sperm count decrease rapidly with repeated ejaculation. Even though it takes only one sperm to fertilize the ovum, each milliliter of semen normally contains about 100 million sperm. Reduction in sperm production is associated with infertility: 50% of men with counts of 20–40 million/mL and essentially all of those with counts under 20 million/mL are sterile. The presence of many morphologically abnormal or immotile spermatozoa also correlates with infertility. The prostaglandins in semen, which come from the seminal vesicles, are at high concentrations, but their function in semen is unknown. The causes of male infertility, as well as the underlying mechanisms of sperm in fertilization, are used as clues in developing male contraception (Clinical Box 23–1).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree