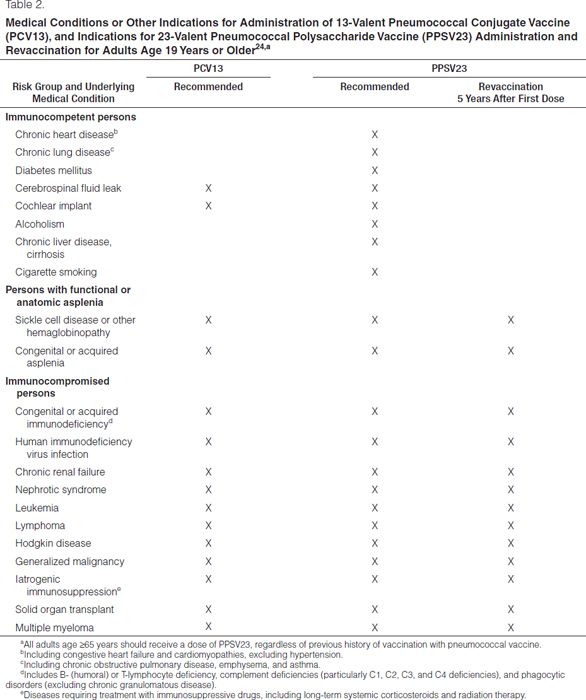
The 23-valent pneumococcal polysaccharide vaccine (PPSV23), the 13-valent pneumococcal conjugate vaccine (PCV13), and the 7-valent pneumococcal conjugate vaccine (PCV7) have been shown to be highly effective in providing protection against the most commonly isolated pneumococcal serotypes that cause human disease, including those serotypes known to be antimicrobial resistant. While the use of these vaccines will not prevent the development of drug-resistant S. pneumoniae, they will likely prevent invasive infection caused by a drug-resistant organism. PPSV23 is recommended for all adults age 65 years or older and other adults with certain comorbid conditions.30 It may also be given to select groups of high-risk patients over 2 years of age (such as those with cochlear implants) as long as it is administered at least eight weeks after administration of the last dose of PCV7 or PCV13. This PPSV23 vaccine does not produce an adequate immunologic response in children under 2 years old and should not be used in that population. PCV7 was added to the standard recommended pediatric vaccines in October 2000.31 PCV13 was added in 2010. The PCV series should be given to all children beginning at age 2 months. A single supplemental dose of PCV13 is recommended for all children age 14–59 months and for children age 60–71 months with certain underlying medical conditions who have already received an age-appropriate PCV7 series.32 The CDC National Immunization Program has established recommendations for pneumococcal vaccine administration.30,32
PPSV23 was licensed in the early 1980s and has demonstrated good immunogenicity in both young and older adults; however, an individual will not develop an immune response to all 23 pneumococcal serotypes.33 The reason for this is unclear. Despite the vaccine’s long-standing availability and documented effectiveness, approximately 64% of eligible persons actually receive it.34 One of the Healthy People 2020 goals is for 90% of eligible adults to receive the pneumococcal vaccine.35 This is an excellent opportunity for pharmacists to improve the vaccination rate of older adult patients and help promote national health care goals.
Revaccination with PPSV23 is recommended for adults over age 65 years if the first dose was administered when they were under 65 years old at the time of vaccination and at least 5 years has elapsed since the first dose.30 In addition, persons younger than 65 years with immunocompromising conditions may receive a one-time revaccination if 5 years has elapsed since the first dose of PPSV23. Revaccination with PPSV23 results in a somewhat blunted immune response and is associated with increased injection-site reactions.33
PCV7 for children has been available since February 2000. Use of this vaccine has been shown to decrease the carriage rate of S. pneumoniae and the incidence of invasive pneumococcal disease, acute otitis media, and pneumonia in vaccinated populations.36,37 The rate of vaccine-specific serotype S. pneumoniae carriage in vaccinated children remains below the rate for nonvaccinated children over prolonged periods of time. Widespread vaccination of children with PCV7 has shown a “herd effect” in decreasing the carriage rate of S. pneumoniae in children, who are an important vector for the transmission of S. pneumoniae to other children and adults.36 This is similar to what was seen with widespread use of the Haemophilus influenzae type B vaccine and the marked decrease in H. influenzae-related infections.
PCV13 for children and adults has been available since February 2010 and has taken the place of the discontinued PCV7. All children age 2–59 months should have routine immunization with PCV13.32 CDC has established routine and catch-up schedules for vaccination. Recommendations by the CDC Advisory Committee on Immunization Practices for the use of PCV13 in adults were established in 2012.30 Patients who have immunocompromising conditions should receive PCV13 in addition to PPSV23 (Tables 1 and 2). Screening and vaccination should occur in all appropriate adult patients. Timing of PCV13 and PPSV23 administration is important to ensure maximal immune response. If the person has never received a dose of PCV13 or PPSV23, he or she should receive a dose of PCV13 followed by a dose of PPSV23 at least eight weeks later. If the patient has received one or more doses of PPSV23, the patient should receive one dose of PCV13 at least one year after the last dose of PPSV23.
Health care institutions should develop robust programs to increase pneumococcal vaccination rates as pneumococcal disease continues to contribute to a significant amount of morbidity and mortaility in the United States. More than 65% of patients with severe pneumococcal disease have been hospitalized in the preceding—three to five years, but few have received vaccine.34 Most people who succumb to preventable infections have visited a health care provider in the preceding year but were not vaccinated.38–42 Clinicians should ensure that patients receive proper immunizations,43,44 and pharmacists can promote vaccination efforts by serving as educators, facilitators, and vaccinators. Currently, all 50 states allow pharmacists to administer vaccines, and 49 states allow pharmacists to administer pneumococcal vaccines.45 Variability exists among the states regarding patient age limitations and pharmacist-administered vaccines, with 13 states authorizing pharmacists to vaccinate patients of any age.46 Although vaccinations are typically administered in the ambulatory care setting, clinicians must also seize opportunities to vaccinate hospitalized patients (Figure 1). Pharmacists have demonstrated that they can increase the number of persons receiving needed vaccines in both inpatient and ambulatory care settings.47
Summary
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


