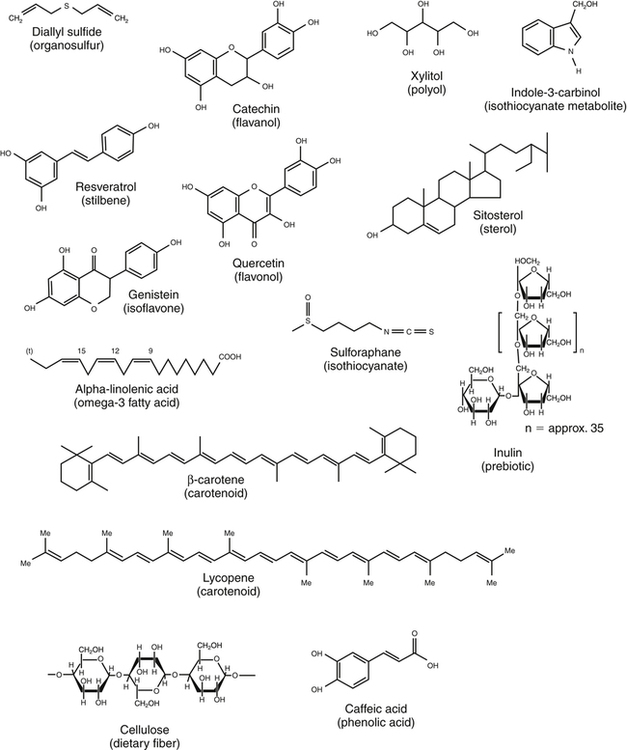
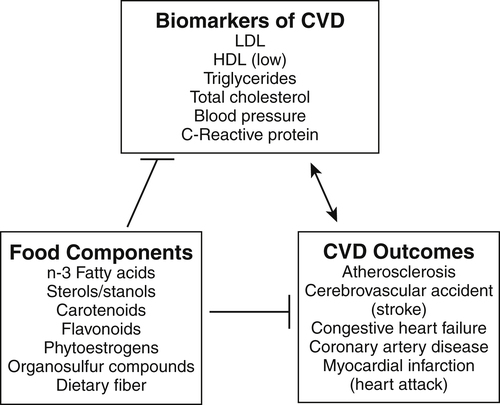
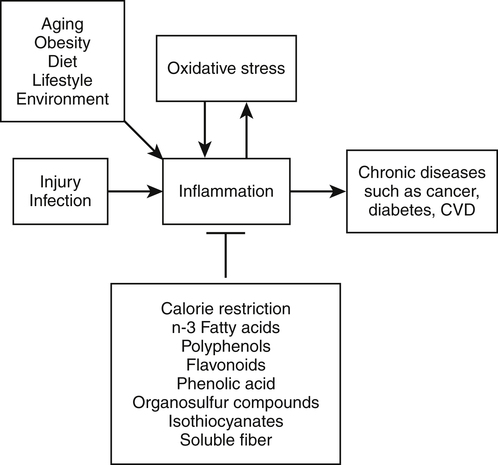
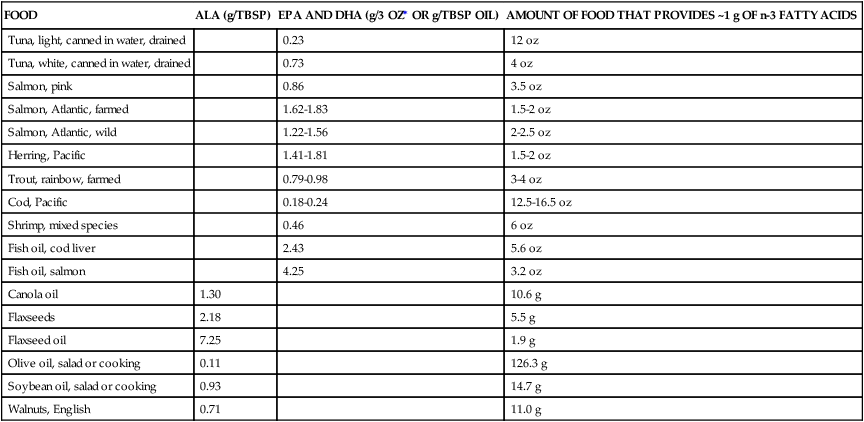
Elizabeth H. Jeffery, PhD, Kelly A. Tappenden, RD, PhD and Anna-Sigrid Keck, PhD, CIP Many bioactive food components have been purified and characterized chemically (Figure 2-1). When studied in purified form, most of these compounds exhibit specific biochemical actions, giving rise to the term nutraceutical, which derives from the idea of a “druglike” nutrient or food component. However, although the study of purified components is frequently used to study mechanism, studies with purified components do not always reflect the full physiological effect of a whole food within the diet (Canene-Adams et al., 2007). This may be due to the presence of multiple compounds in a single food, or the bioavailability of the compound when it is provided as a purified component may be very different than when it is provided within its original food matrix. Foods rich in bioactive food components have been termed functional foods. Although there is no universally accepted definition of a functional food, the International Food Information Council Foundation defines functional foods as those foods that provide health benefits beyond basic nutrition (www.foodinsight.org/Resources/Detail.aspx?topic=Background_on_Functional_Foods). The content of bioactive components in a given food varies with genotype of the plant or animal and the growing, storage, and processing conditions. Extracts or concentrates sold as dietary supplements may be labeled with analytical information, but such labeling is not currently required. Supplements are typically advertised to “maintain health,” such as normalizing blood pressure, whereas researchers more typically use the term disease prevention to describe the same action. Use of the term dietary disease prevention is not limited to maintenance of health but may also refer to the prevention of disease recurrence following successful therapy or the prevention of chronic disease progression, such as bone loss or growth of slow-growing tumors (Mehta et al., 2010). For example, research suggests a possible role for green tea components in slowing increases in white blood cell count in chronic lymphocytic leukemia (Zhang et al., 2008) and a role for tomatoes in slowing increases in prostate-specific antibody, a biomarker for slow-growing prostate cancer (Bowen et al., 2002). Of course for diseases that are aggravated by poor food choices, other aspects of the diet should also be addressed. The FDA allows manufacturers of dietary supplements to state health claims and qualified health claims for their products based on the same approval process required for health claims on conventional foods (see Chapter 3 for further explanation of these claims). In addition, under provisions of the DSHEA, dietary supplements may also carry “structure/function” claims that have not been preapproved by the FDA. Because conventional foods are now allowed to carry these structure/function claims, some foods are also marketed as dietary supplements. However, foods may be marketed as dietary supplements only if it is not a product designed to substitute for a food in a meal rather than being used to supplement the diet. For example, a spread that is used in place of butter could not be marketed as a dietary supplement. For the disease prevention field to move forward, there is a dire need for accurate surrogate endpoint biomarkers of efficacy of foods or bioactive food components in disease prevention in humans. A useful biomarker should be closely linked to the progressive pathway for the disease and correlate with changes in risk (Bowen, 2005). Plasma cholesterol is an example of a well-proven and established biomarker that the public and medical professionals rely on to understand the health status of a person. Plasma cholesterol is a reversible measure of risk for CVD and is used by science researchers for testing hypotheses, as well as by consumers monitoring the impact of a change in diet on risk factors for CVD. Cholesterol and other biomarkers have aided the identification of foods that prevent CVD (Figure 2-2). On the other hand, tools are not readily available for the assessment of risk for cancer, inflammation, and many other chronic diseases. One potential biomarker of hormone-dependent cancer risk in women is the pattern of urinary estrogen metabolites, which is altered by indole-3-carbinol from cruciferous vegetables. Although the mechanism is still under study, increased levels of urinary 2-hydroxyestradiol correlate with a lower risk for breast and uterine cancer. Chronic inflammation, often as a result of obesity, increases risk for many chronic diseases, including cancer. Bioactive food components decrease inflammation via multiple mechanisms, including activation of the transcription factor Nrf2 and epigenetic regulation of gene expression (Thimmulappa et al., 2006; Szarc vel Szic et al., 2010; Kim et al., 2009; Figure 2-3). Research, followed by public education, is needed to develop robust biomarkers in humans for use by consumers and medical professionals in evaluating the impact of diet on disease prevention. The family of tetraterpenes, or carotenoids, contains both vitamin A precursors (α- and β-carotene and β-cryptoxanthin) and a number of other compounds that lack pro–vitamin A activity (including lycopene, lutein, and zeaxanthin). The structures of β-carotene and lycopene are shown in Figure 2-1. The various carotenoids are considered to have potential for prevention of chronic disease. A number of epidemiological studies have found correlations of plasma β-carotene with reduced disease risk. However, some β-carotene intervention studies have found enhanced risk with supplementation. Several explanations of the discordance of research findings are possible. The apparent association of plasma β-carotene with reduced disease risk in the epidemiological studies could be simply because the plasma β-carotene level is an indicator of dietary fruit and vegetable intake. Exposure to other common plant food components such as fiber or other phytonutrients could be responsible for the health benefits. On the other hand, the adverse effects of β-carotene supplementation may be related to total dose or exposure. The β-Carotene and Retinol Efficacy Trial (CARET) reported plasma β-carotene levels of about 2 to 6 μmol/L and the Alpha-Tocopherol, Beta-Carotene Cancer Prevention (ATBC) Trial produced levels of about 4 to 8 μmol/L (Erdman et al., 2010; Greenwald, 2002); both of these studies showed adverse effects. In contrast, the Linxian trial and the Physicians’ Health Study, which yielded plasma β-carotene levels of about 2 μmol/L or less, did not show adverse effects. Nonsupplemented individuals typically have plasma β-carotene levels of less than 1 μmol/L. Another potential explanation might be that high intakes of β-carotene adversely affect intakes of other lipid-soluble nutrients, but this does not appear to be the case. Plasma lycopene, lutein, zeaxanthin, retinol, and α-tocopherol were unaffected by a tenfold increase in plasma β-carotene (Mayne et al., 1998). The unanticipated outcomes of the β-carotene intervention trials is a reminder that whole foods and food components are not the same thing, that associations do not imply a causal relationship, and that dose or exposure matters. Lycopene accumulates preferentially in a few tissues, including prostate. Evidence is accumulating that tomatoes and tomato products rich in lycopene may slow or prevent prostate cancer. There is a qualified health claim associated with lycopene and prostate health (Fleshner and Zlotta, 2007). It remains to be determined if lycopene is effective alone, or possibly requires other components from tomatoes (Canene-Adams et al., 2007). In plasma, lycopene is mostly associated with low-density lipoproteins (LDLs), whereas lutein and zeaxanthin binding is shifted toward high-density lipoproteins (HDLs) (Carroll et al., 2000). In the U.S. population, lycopene is the predominant plasma carotenoid. Lutein and zeaxanthin, but not other carotenoids, accumulate in the macula lutea region of the retina, where they may act as photoprotectors, inhibiting ultraviolet (UV)-induced oxidative damage. One study found a greatly reduced incidence of macular degeneration in those individuals with plasma lutein/zeaxanthin levels in the highest quartile (Bone et al., 2001). The Carotenoids in Age-Related Eye Disease Study (CAREDS) found that lutein and zeaxanthin may protect against macular degeneration in women less than 75 years of age (Seddon et al., 1994; Bone et al., 2001). Until further studies on safety are conducted, foods rich in carotenoids, such as spinach and corn, may provide a safer alternative to taking purified dietary supplements. Five servings of fruits and vegetables a day contain about 6 mg of carotenoids (Ghiselli et al., 1997), which is considered sufficient to provide any possible carotenoid-related health benefits in preventing chronic disease. A typical Western diet contains about 6 mg/day of carotenoids, but about 60% of this is derived from animal sources, which are lower in lutein and zeaxanthin than fruits and vegetables. Good dietary sources of all carotenoids are yellow and orange fruits and vegetables and dark green leafy vegetables. Tomatoes and watermelon are good sources of lycopene. Spinach is a good source of lutein and zeaxanthin as are marigold petals, which are used as a source of these carotenoids for dietary supplements. Bioavailability of all carotenoids is enhanced by the presence of fat in the diet because carotenoids are fat soluble. Polyunsaturated fatty acids (PUFAs) are essential components of our diets as both n-3 and n-6 PUFAs are required for growth, reproduction, and prevention of deficiency diseases (see Chapter 18). Certain n-3 PUFAs have been identified as possibly lowering the risk for chronic disease (Ruxton et al., 2007), and conjugated linoleic acids (CLAs) may also provide health benefits beyond basic nutrition (Bhattacharya et al., 2006). (See Chapter 6 for information about fatty acid structure and nomenclature.) The n-3 fatty acids include α-linolenic acid (C18:3, or ALA), eicosapentaenoic acid (C20:5, or EPA), and docosahexaenoic acid (C22:6, or DHA). ALA is present in plant-based food products such as canola oil, flaxseed, and walnuts (Table 2-1). ALA can serve as a precursor to EPA and DHA, but humans metabolize only about 10% of dietary ALA to EPA and DHA. In the United States, typical intake of ALA is much higher than that of EPA and DHA, unless the diet is supplemented with a good source of the C20 and C22 n-3 fatty acids such as wild-caught (not farmed) oily fish and fish products or fish oil capsules. Cow’s milk may also contain EPA and DHA, depending on bovine genotype and diet. In Iceland, where cows are fed diets that include fish oils, cow’s milk is an excellent source of EPA (Thorsdottir et al., 2004). TABLE 2-1 Abundant Dietary Sources of the n-3 Fatty Acids ALA, DHA, and EPA ALA, α-Linolenic acid; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid. Data from U.S. Department of Agriculture, Agricultural Research Service. (2010). USDA National Nutrient Database for Standard Reference, Release 23. Retrieved from www.ars.usda.gov/ba/bhnrc.ndl Studies suggest that consumption of fish and fish products rich in n-3 PUFAs may provide protection against CVD, cataracts, depression, immune function diseases, and cancer. The strongest association between disease prevention and dietary n-3 PUFAs is for reduction in risk for CVD. A 30-year study of middle-aged men showed an inverse relationship between fish consumption and death from CVD (Daviglus et al., 1997). In the Lyon Diet Heart Study, adults consuming a “Mediterranean” diet high in canola and olive oils had significantly lower blood cholesterol and triglyceride levels, higher HDL cholesterol level, and a 70% reduction in coronary events and cardiac deaths compared to the control group that consumed a normal American diet low in ALA (de Lorgeril et al., 1999). A recent systematic review of the evidence concludes that there is moderate evidence for a relationship between protection against CVD and intake of fish or n-3 PUFAs (Mente et al., 2009). The n-3 PUFAs may also protect against a number of other chronic diseases. For example, changes in mental health may correlate with low dietary intake of n-3 PUFAs, including incidence of depression, although more studies are needed to clarify this (Hallahan and Garland, 2005; Sinn et al., 2010). Inflammatory conditions, including asthma and inflammatory bowel disease, may be abated or prevented by consumption of fish, but there are insufficient data to support a recommendation for the use of EPA/DHA for these purposes (Chapkin et al., 2009). Similarly, although cell culture studies support a role for n-3 PUFAs in prevention of prostate cancer, this is not supported by clinical findings (Kristal et al., 2010). The health benefit of dietary n-3 PUFAs may depend not only on the amount in the diet but also on the ratio of n-6 to n-3 PUFAs. Between a 5:1 and 1.8:1 ratio of n-6 to n-3 PUFAs was associated with a decreased risk for colon cancer (Roynette et al., 2004; Simonsen et al., 1998). A typical American diet provides more n-6 PUFAs than n-3 PUFAs, with a typical ratio of n-6 to n-3 greater than 10:1. This is due to the high seed oil content in the U.S. diet; the ratio of n-6 to n-3 PUFAs in corn oil is 46:1, and it is 7:1 in soy oil. One possible explanation for the importance of this ratio from a health perspective is competition between substrates for enzymes involved in prostaglandin synthesis. For example, dietary linoleic acid is a precursor of arachidonic acid, the n-6 C20 PUFA that serves as substrate for prostaglandin E2 synthesis. Linoleic acid, the n-6 precursor of arachidonic acid, has been associated with stimulation of tumor growth, whereas the n-3 EPA is metabolized to prostaglandin E3, a compound shown to lower tumor growth in vitro. Further research is needed to reach significant scientific agreement about the balance of positive and negative health effects from dietary fish or other sources of n-3 PUFAs. The American Heart Association recommends consuming fish rich in n-3 PUFAs twice a week and choosing oils and foods that are rich in ALA (American Heart Association, 2010). Concerns over heavy metal and toxin contamination of wild fish caution against recommending a higher intake of fish. Some species of wild fish may contain high levels of environmental contaminants, including methyl mercury, dioxins, and polychlorinated biphenyls. Levels of these substances are low in fresh and sea waters, but they accumulate in the food chain; hence bigger and older fish contain the highest levels. In the United States, the Environmental Protection Agency and the FDA are responsible for sport-caught fish and commercial fish, respectively. These federal agencies provide consumer information on mercury levels in fish. There is some evidence suggesting that CLAs protect against CVD and certain cancers. CLAs may lower the risk for atherosclerosis by altering the metabolism of very-low-density lipoprotein cholesterol (McLeod et al., 2004). Protection against cancer by CLAs has been reported in animal models, but clinical studies are not definitive (Bhattacharya et al., 2006; Voorrips et al., 2002). It should be noted that these naturally occurring CLA with trans bonds are very different than the trans isomers of fatty acids that are formed as a result of hydrogenation or partial hydrogenation of vegetable oils. Although there are limited data in humans, the intake of trans fatty acids in hydrogenated fats has been suggested to increase LDL cholesterol, decrease HDL cholesterol, and promote both CVD and cancer growth (de Roos et al., 2003; Benatar, 2010). More than 200 sterols and stanols have been identified in plants. The most abundant sterols are β-sitosterol, stigmasterol, and campesterol, whereas the most abundant stanols are sitostanol and campestanol (Moreau et al., 2002). Sterols are essential components of cell membranes. All sterols contain the sterol ring structure but differ in the side chain (see structure of sitosterol in Figure 2-1). Stanols are the saturated form of sterols, with no double bond in the sterol ring. The extent of phytosterol absorption from the intestine is 10% to 15% for campesterol and campestanol, 4% to 7% for sitosterol, and about 1% for sitostanol, compared to 50% for a bolus of cholesterol (Katan et al., 2003). Cholesterol, the most abundant sterol in animals, is not synthesized in plants. The cholesterol-lowering effect of plant sterols was first identified in the 1950s. Plant stanol and sterol esters lower plasma cholesterol by reducing intestinal cholesterol absorption. More than 50 trials suggest that 1.5 g/day or more of stanol and sterol esters can decrease LDL cholesterol by 10% (Katan et al., 2003). There appears to be little additional benefit from ingesting larger amounts. The addition of stanol or sterol esters is also effective in patients already on a heart-healthy diet or in those taking cholesterol-lowering medications. In one study, the addition of 2.3 g/day of stanol esters to the diet of patients following the National Cholesterol Education Program (NCEP) Step I diet produced an 8% to 11% greater decrease in total cholesterol and a 9% to 14% greater decrease in LDL cholesterol than was observed in control subjects (Hallikainen and Uusitupa, 1999). Many studies have shown that stanols and sterols can provide a further cholesterol-lowering effect in hypercholesterolemic patients taking statin medications. In one such study, intake of 3 g/day of stanol esters resulted in an additional 10% reduction in LDL cholesterol levels compared to statins alone (Blair et al., 2000); this improvement was substantially better than that obtained by doubling the statin dose, which would be expected to decrease plasma LDL cholesterol by an additional 6%. Emerging evidence indicates that plant sterols and stanols may have other biological roles, including the reduction of various forms of cancer (Jones and AbuMweis, 2009). Mounting evidence indicates that phytosterols inhibit growth of various forms of lung, stomach, ovarian, and breast cancer. Mechanisms identified include inhibition of carcinogen activation, cancer cell growth, angiogenesis, invasion and metastasis, and apoptosis of cancerous cells. Phytosterol consumption may also increase antioxidant enzyme activities and thereby reduce cellular damage induced by oxidative stress. Plant stanol and sterol esters are considered to be generally recognized as safe (GRAS) by the FDA, and phytosterol ester margarine has been determined to be safe by the Scientific Committee on Foods of the European Union. A meta-analysis of 41 studies led to the conclusion that consumption of phytosterols by healthy humans at the level of 2 g/day is not associated with any major health risks (Katan et al., 2003). Polyphenolics constitute a broad category of secondary plant compounds including simple phenols as well as highly polymerized compounds with molecular masses greater than 30 kDa; the most common forms in food are the flavonoids and phenolic acids. Typically polyphenolics are present in their free (i.e., aglycone) form or as O-glycosides (Bravo, 1998). The biological characteristic that brings this broad group of more than 8,000 different compounds together is the hypothesis that the hydroxyl groups might provide reducing power or “antioxidant potential” that might protect the body from oxidative damage due to reactive oxygen species (ROS). This has led to the chemical evaluation of the radical “quenching power” of purified polyphenolics and plant extracts. Flavonoids are the most prominent subclass of dietary polyphenolics (Beecher, 2003). Chemically, the flavonoids are low-molecular-weight diphenylpropanes consisting of two aromatic rings joined by a 3-carbon chain that most frequently incorporates oxygen into a cyclic arrangement to form a third interconnecting ring (see catechin, quercetin, and genistein in Figure 2-1). Common flavonoid subclasses are flavones (e.g., apigenin and luteolin), flavonols (e.g., quercetin and kaempferol), isoflavones (e.g., genistein and daidzein), flavanols (e.g., catechins and epigallocatechin gallate), flavanones (e.g., naringenin), and anthocyanidins (e.g., cyanidin and petunidin). Proanthocyanidins, sometimes called tannins, are oligomers or polymers of flavanol units. Flavonoids are present in most plant-based foods, with the majority of dietary flavonoids being derived from teas, berries, other colorful fruit, red wine, and dark chocolate. In addition, flavonoids may be key bioactive components in several herbs used as dietary supplements, such as ginger, licorice, ginseng, kava kava, ginkgo biloba, and St. John’s wort. Flavonoids have been proposed to exert a wide variety of biological activities in humans, including antiinflammatory, antioxidative, antiallergic, and anticarcinogenic activities (Kostyuk and Potapovich, 1998; Guo et al., 2009; Jagtap et al., 2009). Epidemiological studies support a role for flavonoids in prevention of both CVD and cancer (Hertog et al., 1995). In addition, both clinical and animal studies have suggested an inverse relationship between intake of flavonoids and the incidence of a number of cancers. In a cohort study of 10,000 Finnish men and women, diets that were high in flavonoids (e.g., apples and onions) were associated with a 20% lower incidence of cancer at all sites and a 46% decrease in incidence of lung cancer in particular (Knekt et al., 1997). Animal studies showed a cancer preventive effect of green tea or pure epigallocatechin-3-gallate (Yang et al., 2009). A double blind placebo control study showed that 1 g/day of quercetin for 12 weeks reduced severity of upper respiratory tract infection by 36%, resulting in 31% fewer sick days in a group of middle-aged and older subjects who rated themselves as physically fit (Heinz et al., 2010). Licorice extracts rich in flavonoids may provide protection against peptic ulcer or gastric cancer by inhibiting growth of Helicobacter pylori in humans (Fukai et al., 2002). Whereas free flavonoids can be absorbed across the small intestinal mucosa (Manach et al., 1997), flavonoid glycosides require hydrolysis by digestive enzymes and/or the colonic microbiota to release the flavonoid before it can be absorbed (Hollman and Katan, 1997). After absorption, flavonoids undergo methylation and/or conjugation to glucuronic acid or sulfate before excretion. Conjugates excreted in the bile undergo deconjugation in the gut, catalyzed by gut microbiota, and the flavonoid may be reabsorbed (Manach et al., 1997). Many published studies indicate that flavonoids are only partially absorbed, depending on both the compound and the food matrix. For example, 52% of quercetin in quercetin glycoside from onions was absorbed, whereas only 24% of a pure test sample of free quercetin was absorbed (Hollman and Katan, 1999). Disposition and excretion also depend on both the form of the compound and on other components in the food or meal. For example, tea catechins reached maximum blood levels 2 hours after ingestion of either black or green tea, but green tea catechins were eliminated more rapidly with the half-life for green tea catechins being 4.8 hours compared to 6.9 hours for black tea catechins (van het Hof et al., 1998). Intake of total flavonoids ranges from about 20 mg/day in the United States, Denmark, and Finland to about 70 mg/day in Holland and Japan (Beecher, 2003). There are two excellent databases of flavonoid content of foods: the USDA Database for the Flavonoid Content of Selected Foods (USDA, 2007) and Phenol-Explorer (Neveu et al., 2010). Several observations suggest caution in the use of flavonoid supplements. First, as previously seen with β-carotene, flavonoids may act as antioxidants at one dose but as prooxidants at a higher dose (Babich et al., 2008). Second, bioactive food components that are potentially anticarcinogenic in adults may be associated with adverse effects in the developing fetus. For example, pregnant women ingesting unusually large amounts of foods containing topoisomerase II inhibitors, which include flavonoids in soy, coffee, wine, tea, and cocoa, may have a greater risk for giving birth to a child with acute myeloid leukemia (Ross, 1998).
Food Components with Health Benefits
Functional Foods and Dietary Supplements
Definitions of Functional Foods and Dietary Supplements
Regulations Affecting Dietary Supplements
The Need for Surrogate Endpoint Biomarkers of Efficacy
Carotenoids
Polyunsaturated Fatty Acids
FOOD
ALA (g/TBSP)
EPA AND DHA (g/3 OZ∗ OR g/TBSP OIL)
AMOUNT OF FOOD THAT PROVIDES ~1 g OF n-3 FATTY ACIDS
Tuna, light, canned in water, drained
0.23
12 oz
Tuna, white, canned in water, drained
0.73
4 oz
Salmon, pink
0.86
3.5 oz
Salmon, Atlantic, farmed
1.62-1.83
1.5-2 oz
Salmon, Atlantic, wild
1.22-1.56
2-2.5 oz
Herring, Pacific
1.41-1.81
1.5-2 oz
Trout, rainbow, farmed
0.79-0.98
3-4 oz
Cod, Pacific
0.18-0.24
12.5-16.5 oz
Shrimp, mixed species
0.46
6 oz
Fish oil, cod liver
2.43
5.6 oz
Fish oil, salmon
4.25
3.2 oz
Canola oil
1.30
10.6 g
Flaxseeds
2.18
5.5 g
Flaxseed oil
7.25
1.9 g
Olive oil, salad or cooking
0.11
126.3 g
Soybean oil, salad or cooking
0.93
14.7 g
Walnuts, English
0.71
11.0 g
Plant Sterols/Stanols
Polyphenolics
Flavonoids
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Basicmedical Key
Fastest Basicmedical Insight Engine