Follicular Lymphoma
Definition
Follicular lymphomas (FLs) are composed of cleaved and noncleaved B cells recapitulating the germinal centers of lymphoid follicles and growing in a nodular pattern.
Synonyms
Nodular lymphosarcoma; Germinal center lymphoma; Nodular (and/or diffuse) lymphoma: poorly differentiated lymphocytic, mixed poorly differentiated lymphocytic and histiocytic, histiocytic, or undifferentiated (Rappaport) (1); Centroblastic–centrocytic follicular; centroblastic follicular; follicular and diffuse lymphoma (Kiel) (2); Small cleaved, large cleaved, small noncleaved, large noncleaved follicular lymphoma (Lukes-Collins) (3); Follicular lymphoma, predominantly small cleaved cell, mixed small- and large-cell, predominantly large-cell (Working Formulation) (4); Follicle center lymphoma, follicular, diffuse (REAL) (5); Follicular lymphoma (WHO) (6).
Epidemiology
In the United States, FL is the second most common non-Hodgkin lymphoma, representing 22% of 1,378 cases in a multi-institutional study (7) and 70% of the low-grade lymphomas in American and European clinical trials (8). It is uncommon in Asia and underdeveloped countries (9). The male predominance noted in other lymphomas is not observed in FL, in which the male-to-female ratio is 1:1.7 (4,7). The median age is 59 years (7). Follicular lymphoma is a neoplasm of older age seen in only 1% to 2% of lymphoma patients before the age of 20 years (7,10,11). Follicular lymphoma in children is usually limited to lymph nodes or tonsils. It pursues an indolent clinical course, rarely progressing to a higher grade or a diffuse pattern and, unlike FL in adults, it can be cured with conservative therapy (11).
Pathogenesis
A germinal center origin, low proliferation rate, and nodular growth pattern characterize FLs. The complex architectural relationship between FL cells and accompanying non-neoplastic T cells and follicular dendritic reticulum cells (DRCs) suggests a continuing response to local signals. Immunoglobulin heavy and light chains are rearranged. The translocation t(14:18) affecting BCL-2 is present in 90% of FL grades 1 and 2 but in only about 70% of grade 3. Although the t(14:18) chromosomal translocation is associated with overexpression of antiapoptotic BCL-2 protein in most FLs, it is not sufficient to produce lymphomagenesis, as demonstrated by the finding of t(14:18) in some lymphocytes from normal persons (12). Moreover, the translocation t(14:18) is the sole genetic abnormality in only 10% of cases, which indicates that other changes are necessary for the transformation to lymphoma (13,14,15). These are additional chromosomal breaks, a median of six in one study, which are associated with a worse prognosis (13). In addition to BCL-2 protein, FLs express other cell death suppressor proteins belonging to the BCL-2 family (16), such as BCL-xL and MCL1 (17). Follicular lymphomas, unlike germinal center B cells, produce very low or undetectable amounts of the cell death-promoting proteins BAX and BAD (17). Eight proteins that are inhibitors of apoptosis (IAP) have been identified in humans, some of these, such IAP1, are present in over 90% of FLs (18). Differences of expression of IAPs and acquisition of novel mutations may explain their further transformation to diffuse or higher grade forms of lymphoma (19).
More recently, the role of the immunologic microenvironment, particularly T cells and dendritic cells, in determining the clinical behavior and prognosis of FL has been revealed (20,21). CD40 ligand positive (CD40L+) T cells that reside in secondary follicles can inhibit FL cell death, and follicular dendritic cells (DRC) can interact with FL cells to prevent their apoptosis (22,23,24).
Clinical Syndrome
Follicular lymphoma is an indolent disease in which B-type symptoms are noted in only 20% of patients (25). Because of its insidious onset, about two-thirds to three-fourths of patients are already in stage III or IV at the time of diagnosis (25,26,27). Follicular lymphoma is less likely than the diffuse lymphomas to involve extranodal primary sites; however, it is almost always disseminated at the time of diagnosis, often involving bone marrow, liver, and abdominal lymph nodes. The bone marrow is frequently involved, particularly in grades I and II of FL, resulting in a characteristic paratrabecular infiltrate. In a study of 450 non-Hodgkin lymphomas, the bone marrow was infiltrated most commonly, 30.4%, by FL grade 1, and far less often by large-cell lymphomas (28). Because of this tendency, the bone marrow examination is a routine staging procedure in FL (29). The incidence of bone marrow disease rises with the number of involved lymph node groups (30). Only a minority of patients present in clinical stage I or II. The proportion of patients with FL, grade 1 who have stage IV disease is greater than the proportion of those with grades 2 and 3 who have stage IV disease (25,26,27). Cervical and inguinal lymph nodes are involved most often. Mediastinal lymph nodes are very rarely a primary location.
The Ann Arbor staging system predicts long-term prognosis poorly in FL because many patients already have widely disseminated disease at diagnosis, and lymphoma cells appear in the peripheral blood (26). Factors that adversely affect complete remission and survival include an increased number of involved lymph node groups, constitutional symptoms, splenomegaly, and older age (30).
The histologic grades correlate with prognosis, with grades 1 and 2 being indolent whereas grade 3 has high risks for early relapse and death (31). Progress in FL correlates with transformation to diffuse large-cell lymphoma, analogous to the Richter syndrome that occurs in up to 30% of cases (32,33). The transformation is associated with alteration in p53 and other deletions and alterations of chromosome 9 (34,35). The transformation to diffuse large-cell forms of lymphomas is
usually associated with resistance to treatment and altered clinical course (32,36).
usually associated with resistance to treatment and altered clinical course (32,36).
The clinical course of FL is characterized by its initial responsiveness to treatment followed by repeated relapses and final progression to diffuse large-cell lymphoma. The survival curves for all histologic subtypes of FL have a similar shape, without a plateau, which suggests that current therapy has no curative potential (25,26,27). Patients with early-stage FL, especially if young, can have a good result after radiation therapy (37). Generally, they experience long survivals but few are cured (38). The spectrum of clinical outcomes in FL is broad; about one-fifth of patients die within 2 years, and about one-fifth are alive 15 years after diagnosis (26). The grading of histologic type should be supplemented by clinical information, such as the International Prognostic Index (IPI) (38), when clinical behavior is predicted. The overall and failure-free survival of FL patients with a high IPI is poor (7).
Overall survival at 10 years is up to 79% (39). For those with high-grade, extensive disease or transformation to diffuse large-cell lymphoma, the addition of the anti–B-cell monoclonal antibody rituximab has been the most successful recent lymphoma treatment (40).
Histopathology
The normal lymph node architecture is obliterated by numerous nodules (Fig. 64.1) of fairly uniform size and shape that involve the cortex and medulla and frequently penetrate the perinodal fibroadipose tissues (Fig. 64.2). The neoplastic nodules may occupy the lymph node totally or partially. When partially occupied, the lymph node areas lacking the nodular pattern represent either residual, uninvolved parenchyma or lymphoma of diffuse pattern resulting from the progression of nodular lymphoma (41).
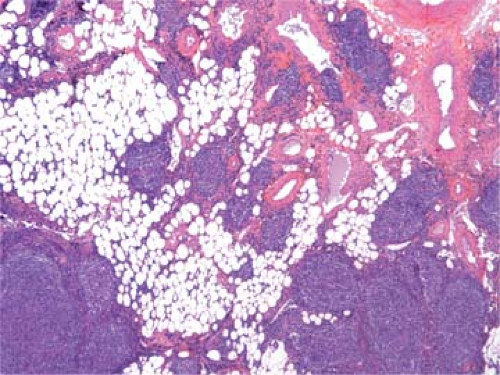 Figure 64.2. Follicular lymphoma. Extension of neoplastic nodules into the perinodal adipose tissue. Hematoxylin, phloxine, and saffron stain. |
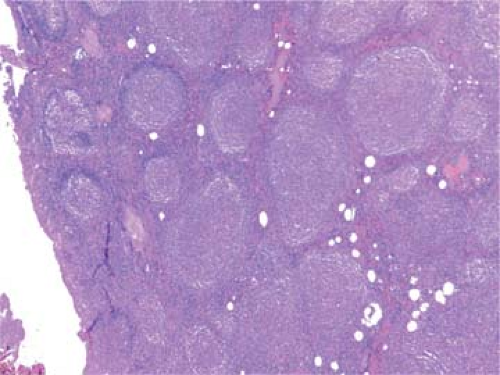 Figure 64.3. Follicular lymphoma composed of homogeneous nodules lacking tingible-body macrophages and mantle zones. Hematoxylin, phloxine, and saffron stain. |
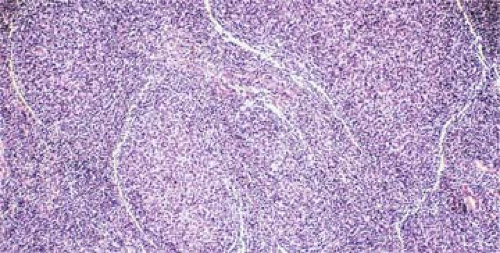 Figure 64.4. Follicular lymphoma. Artifactual cracking around nodules is a clue that neoplastic follicles are not intrinsically part of the lymph node architecture. Hematoxylin and eosin stain. |
In contrast to the enlarged lymphoid follicles of reactive follicular hyperplasia (RFH), which vary greatly in size and shape and frequently coalesce, the nodules of FL are round, isolated, and uniform (Fig. 64.3). Frequently, the neoplastic nodules of FL lack a mantle zone, so that the borders are blurred (see Fig. 64.3), but usually the nodules are sharply circumscribed. Sometimes, a “cracking” artifact highlights the neoplastic nodules (Fig. 64.4). In comparison with the follicles of RFH, the
nodules of FL are more numerous and closer to each other, with very little uninvolved tissue left between them (Figs. 64.1 and 64.3). The nodules are not restricted to subcapsular areas but also involve the deep cortex and medulla (Figs. 64.1 and 64.3). The distinctive nodular pattern of FL may not involve the entire lymph node, which may also exhibit diffuse areas constituted by the same type of neoplastic cells. Since the diffuse growth is usually associated with the progression of the lymphoma and therefore with a worsening prognosis, the ratio of follicular versus diffuse cells should be reported as: predominantly follicular (>75% follicular), follicular and diffuse (25% to 75% follicular), and minimally follicular (<25% follicular) (6).
nodules of FL are more numerous and closer to each other, with very little uninvolved tissue left between them (Figs. 64.1 and 64.3). The nodules are not restricted to subcapsular areas but also involve the deep cortex and medulla (Figs. 64.1 and 64.3). The distinctive nodular pattern of FL may not involve the entire lymph node, which may also exhibit diffuse areas constituted by the same type of neoplastic cells. Since the diffuse growth is usually associated with the progression of the lymphoma and therefore with a worsening prognosis, the ratio of follicular versus diffuse cells should be reported as: predominantly follicular (>75% follicular), follicular and diffuse (25% to 75% follicular), and minimally follicular (<25% follicular) (6).
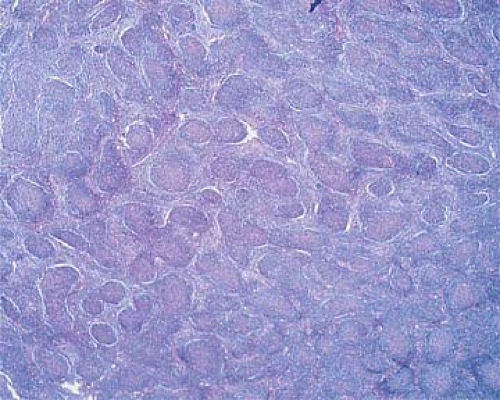
The cells in the nodules are similar to each other, representing a homogeneous, monotonous, monoclonal population (Fig. 64.3). Mitoses are infrequent, and no macrophages containing nuclear debris (tingible-body macrophages) are seen, as they are in the hyperplastic follicles of reactive lymphadenopathies forming the starry-sky pattern. Between the expanding neoplastic nodules, residual lymphocytes and sinus histiocytes appear compressed (Figs. 64.1 and 64.3), whereas vessels and reticulin fibers are distorted, as clearly shown by silver staining. Most characteristic of FLs is their tendency to form nodular structures, even outside the lymph nodes, in the tissues they invade (Figs. 64.2 and 64.5). The invasion of perinodal adipose tissues appears in a nodular form, composed of multiple round aggregates of various sizes spreading out through the fat.
Cytologically, the nodules of FL comprise neoplastic cells recapitulating the germinal centers of lymphoid follicles. Normally, the germinal centers include a spectrum of B-lymphoid cells characterized by cleaved nuclei and varying from small to large centroblasts, which are larger cells with noncleaved nuclei, vesicular chromatin, peripheral nucleoli, and a narrow rim of hyperchromatic cytoplasm, and centrocytes, which are smaller cells with a cleaved, angulated nucleus, dispersed chromatin, small nucleoli, and almost no visible cytoplasm. The nodules of FL are composed of variable proportions of small cleaved and large noncleaved lymphoma cells, similar to those of the normal germinal center. In addition, the normal germinal centers include a large number of T-cells (mostly helper) and a population of supportive, desmosome-connected follicular DRCs.
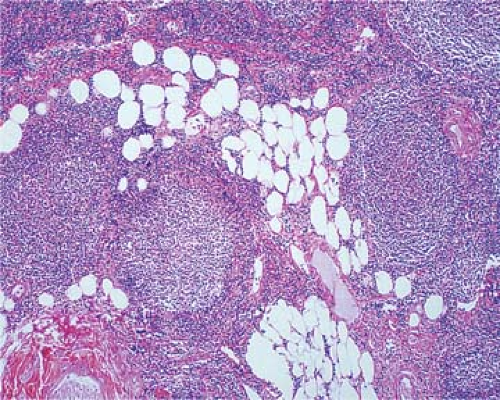 Figure 64.5. Follicular lymphoma maintain the nodular patterns even in the perinodular or other tissues which they invade. Hematoxylin, phloxine, and saffron stain. |
TABLE 64.1 FOLLICULAR LYMPHOMAS: GRADING | |
|---|---|
|
Grading
The grading of FL has prognostic and therapeutic significance. It is based on the count of centroblasts in 10 neoplastic follicles under a × 40 high-power microscopic field (HPF). Grade 1 (0–5 centroblasts/HPF), grade 2 (6–15 centroblasts/HPF), and grade 3 (>15 centroblasts/HPF). If areas of grade 3 are present in an otherwise FL of grade 1 or 2, it should be mentioned separately. Grade 3 can be further subdivided into grade 3a, in which centrocytes are still present, and grade 3b, in which solid sheets of centroblasts are present (6,42) (Table 64.1).
Follicular lymphoma, grade 1 (Table 64.1), predominantly small cleaved cell type (Fig. 64.6), is characterized by cells the size of normal lymphocytes or slightly larger; their angulated, irregularly twisted nuclei have indented or cleaved nuclear membranes and linear streaks representing nuclear infoldings. The nucleoli are small to inconspicuous, multiple, and peripherally apposed to the nuclear membrane.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree