Chapter Twenty. Fluid, electrolyte and acid–base balance
Introduction
Cell function depends on the maintenance of a stable environment through the continuous supply of nutrients, removal of waste and homeostasis of the surrounding fluids. Therefore it is essential that the fluid, electrolyte, acid and base balances of the extracellular fluids be kept within a narrow range. For instance, changes in the composition of electrolytes can affect the electrical potentials of neurons and can move fluid from one compartment to another. Changes in pH can disrupt cellular enzyme systems. Cells also depend on a continuous supply of nutrients and the removal of metabolic wastes. Various organs are involved in coordinating this fluid balance and therefore the purpose of this chapter is to step outside of individual systems and examine the integration of systems in the control of this extremely important aspect of life. Understanding the basic information on biochemistry provided in Chapter 1 will be of benefit to the reader before proceeding with this chapter.
Fluid and electrolytes
Body water content
In an adult, water accounts for about 50% of the body mass although this ratio can vary depending on age, sex, body weight and relative amount of body fat (Marieb & Hoehn 2008). Infants contain approximately 73% of water because of their lower bone mass and body fat. Men contain more water than women because of the extra amount of female adipose tissue and their lower muscle mass. Body fat leads to a reduction in water content as fat is the least hydrated of all body tissues so that obese people contain less water proportionate to their body weight. Older people have less water as their fat content is increased and their muscle content is decreased. Also, as the kidney ages it is less able to concentrate urine so that more fluid is lost in urine. Other losses of body fluid can therefore be life threatening in the elderly. Table 20.1 provides a summary of the distribution of body fluid by weight in a 70 kg man.
| Compartment | Body weight (%) | Volume (litres) |
|---|---|---|
| Intracellular fluid | 40 | 28 |
| Extracellular fluid—interstitial | 15 | 11 |
| Extracellular fluid—intravascular | 5 | 3 |
| Total body water | 60 | 42 |
Fluid compartments
There are three main compartments of the body where water can be found (Fig. 20.1). These are intracellular fluid (ICF), the fluid inside the cells, and extracellular fluid (ECF) which can be divided into interstitial fluid, the fluid between the cells, and plasma, the fluid inside the vascular system. Special types of ECF separate from interstitial fluid and plasma are lymph, transcellular fluid (secreted by cells), synovial, intestinal, cerebrospinal fluid, sweat, urine, pleural, peritoneal, pericardial and intraocular fluid (Martini & Nath 2009). These fluids are usually considered to be part of ECF because of the similarity in composition. The sum of all of the above is the total body water (TBW).
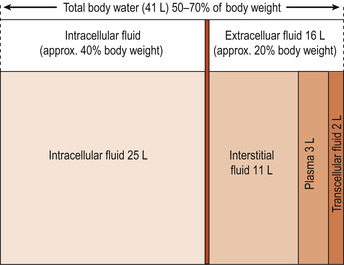 |
| Figure 20.1 Size of the major body fluid compartments. (From Hinchliff S M, Montague S E 1990, with permission.) |
Composition of body fluids
Solutes: electrolytes and non-electrolytes
Water is the universal solvent and contains a variety of solutes. Broadly speaking, these can be divided into electrolytes and non-electrolytes. The non-electrolytes have bonds (usually covalent bonds) that prevent them dissociating into their component particles in solution and therefore do not carry electrical charges. These are mainly organic molecules such as glucose, lipids, creatinine and urea. Electrolytes are chemical compounds that do dissociate into ions in water. They are said to ionise and are charged particles capable of conducting an electric current. Electrolytes include inorganic salts, both inorganic and organic acids and bases and some proteins.
All dissolved solutes contribute to the osmotic activity of a fluid but electrolytes have the greatest osmotic power because each molecule can dissociate into at least two ions. An example is sodium chloride (NaCl):
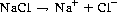
(20.1)
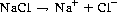
Electrolytes have the greatest ability to cause fluid shifts because water moves along osmotic gradients from areas of lesser osmolality to areas of greater osmolality.
Differences in composition between intracellular fluids and extracellular fluids
Each fluid compartment has its own pattern of electrolytes. Except for the high protein content of plasma, all extracellular compartments have a similar composition. Sodium is the most abundant ECF cation and chloride the major anion. In the ICF, potassium is the most abundant cation and the major anion is phosphate ( 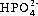 ). The balance in concentrations of sodium in ECF and potassium in ICF reflects the activity of the sodium pump (see Ch. 2).
). The balance in concentrations of sodium in ECF and potassium in ICF reflects the activity of the sodium pump (see Ch. 2).
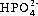 ). The balance in concentrations of sodium in ECF and potassium in ICF reflects the activity of the sodium pump (see Ch. 2).
). The balance in concentrations of sodium in ECF and potassium in ICF reflects the activity of the sodium pump (see Ch. 2).Movement of fluid between compartments
Water movement between plasma and interstitial fluid
The distribution of water and the movement of nutrients and waste products between the plasma in the capillary and the interstitial space occur because of changes in hydrostatic pressure and osmotic forces between the arterial and venous ends of the capillary network. The capillary membrane is semipermeable and allows interchange of fluids and solutes between the intravascular and interstitial fluid (IF) compartments.
The movement of fluid back and forth across the capillary wall is called net filtration (Starling’s hypothesis). The major forces of filtration are within the capillary. Net filtration is the balance between forces favouring filtration, such as capillary hydrostatic pressure (blood pressure) and interstitial oncotic pressure, and forces opposing filtration, such as plasma oncotic pressure. As the plasma flows from the arterial to the venous end of the capillary, blood pressure falls, reducing the hydrostatic pressure. Oncotic pressure remains constant. At the arterial end of the capillary, hydrostatic pressure exceeds oncotic pressure and fluid is forced out into the interstitial space. At the venous end of the capillary, oncotic pressure exceeds hydrostatic pressure and fluid is drawn back into the capillary (Sherwood 2006).
Water movement between ICF and ECF
This water movement between compartments is a function of osmosis. Water moves freely across cell membranes so that the osmolality of TBW is normally at equilibrium. The ICF balance is maintained by active transport of ions out of the cell and interstitial hydrostatic pressure. However, normally, the interstitial forces are negligible because only a very small amount of plasma protein crosses the capillary membrane so that the major forces of filtration are within the capillary. Movements of respiratory gases, nutrients and wastes are unidirectional.
Water balance
Water intake must balance water loss; Table 20.2 summarises the normal daily water balance in a healthy adult.
| Intake | Amount | Output | Amount (ml) |
|---|---|---|---|
| Drinking | 1400–1800 | Urine | 1400–1800 |
| Water in food | 700–1000 | Faeces | 100 |
| Water of oxidation | 300–400 | Skin | 300–500 |
| Lungs | 600–800 | ||
| Total | 2400–3200 | 2400–3200 |
Regulation of water intake
Regulation of water intake is by the mechanism of thirst, which is poorly understood. A thirst centre in the hypothalamus responds to either a drop in plasma volume or an increase in plasma osmolarity. It is probable that the salivary glands, which obtain their fluid from the blood, produce less saliva and the resulting dry mouth makes us drink. Thirst is quenched as soon as we have taken on board the right amount of water, even before there has been time for it to affect blood volume.
Regulation of water output
Water is lost from the body in ways that cannot be avoided. These are the obligatory water losses and explain why we cannot survive long without drinking. They include the insensible loss of water from the lungs and via the skin. Because of the large amount of perspiration lost daily, especially in a hot climate, humans are of necessity a riverine species. That is to say that most settlements before the advent of piped water were next to a river. Water in faeces must be added to the loss. There is an absolute minimum of 500 ml of urine per 24 h that the kidneys must excrete even when the urine is concentrated to its maximum level possible.
Disorders of water balance
Oedema
Oedema is the accumulation of fluid within the interstitial space. It is a problem of fluid distribution and does not necessarily indicate excess intake. Oedema may be accompanied by signs of dehydration if fluid becomes sequestered (locked) within a compartment. It may be caused by factors that increase fluid flow out of the plasma or hinder its return. There are four major contributors to oedema:
1. Increased capillary hydrostatic pressure may occur from venous obstruction such as in thrombophlebitis, hepatic obstruction, tight clothing or prolonged standing.
2. Reduced plasma oncotic pressure follows the loss of plasma proteins found in renal failure, diminished production of plasma proteins found in liver disease or protein malnutrition.
3. Increased capillary membrane permeability is usually associated with inflammatory or immune reactions. Burns, crush injuries, cancer and allergy also produce this effect.
4. If the lymphatic system is blocked by infection or inflammation or lymphatic cancer or has had to be surgically removed in areas to prevent the spread of cancer, proteins and fluids accumulate in the interstitial spaces causing localised lymphoedema.
Clinical manifestations
Oedema may be generalised or localised. It is associated with weight gain, swelling of the tissues and puffiness. Clothing may feel tight. Movement may be limited and blood flow may be restricted. Wounds tend to heal more slowly and the risk of pressure sores and wound infections is increased. The sequestered fluid is not available for metabolic processes and dehydration may occur, for instance following burns. Hypovolaemic shock may occur. Treatment is tailored to fit the individual case and could include elevation of affected limbs, support stockings, avoiding prolonged standing, reducing salt intake and the prescribing of diuretics.
Electrolyte balance
Electrolytes include salts, acids and bases. Salts are the main electrolytes and are involved in many physiological processes. The four main electrolytes are sodium, potassium, calcium and magnesium. Salts are obtained from the food we eat and also, to a lesser extent, in our drinking water. Small amounts of salts may be released during metabolism. An example would be the release of phosphate during the breakdown of nucleic acids.
A major problem for humans is the love of salty food. This may be an acquired taste but is equally as likely to have an innate factor because of the need to replenish salts lost in perspiration. Salts are lost from the body in faeces and urine as well as in perspiration, as mentioned above. If we are depleted of salt our perspiration will be more dilute but, even so, in hot weather a good deal of salt can be lost.
The role of sodium in fluid and electrolyte balance
Salts containing sodium account for at least 90% of solutes in the ECF. Regulating the balance between sodium intake and output is a major function of the kidneys. Sodium is the most abundant cation in the ECF and is the main cause of osmotic pressure. Sodium does not cross cell membranes very easily (Ch. 2) and is therefore ideal for controlling the ECF volume and water distribution in the body. Water follows salt so that a change in sodium content will be followed by a change in water content of a fluid compartment. Blood volume and blood pressure are linked to sodium balance and there is a hormonal regulatory effect by the hormone aldosterone; this is discussed more fully in Chapter 17.
Aldosterone
Aldosterone is produced by the cortical cells of the adrenal gland and its release is mediated by the production of renin by the juxtaglomerular apparatus of the kidney, as explained in Chapter 19. The renin–angiotensin–aldosterone system is also discussed fully in Chapter 19. In brief, renin catalyses a series of reactions leading to the activation of angiotensin II, which causes aldosterone release. Normally, without the influence of aldosterone, about 75% of the sodium in the renal filtrate is reabsorbed in the proximal tubules of the nephrons of the kidneys.
If aldosterone levels are high, most of the remaining sodium is reabsorbed in the distal tubules and collecting ducts. If the permeability of the tubules has been increased by antidiuretic hormone (ADH, also known as arginine vasopressin or AVP), water will passively follow the sodium. There will be sodium and water retention. When aldosterone release is inhibited, there will be little reabsorption of sodium beyond the proximal tubules. Urinary excretion of large amounts of sodium will always result in the excretion of large amounts of water. The effect of aldosterone is to allow large amounts of sodium-free water to be excreted in times of sodium depletion. Like all hormones, aldosterone has a slow effect, taking hours or days to alter fluid compartments.
Other influences on fluid and electrolyte balance discussed in other chapters are the cardiovascular system baroreceptors, the regulation of ADH and the influence of atrial natriuretic factor. Oestrogens and glucocorticoids also play a part in enhancing tubular reabsorption of sodium.
Regulation of potassium balance
Potassium (K +) is the main cation in ICF and is necessary for normal neuromuscular functioning and other processes such as protein synthesis. Potassium is quite toxic, especially to heart muscle. Both hyperkalaemia (excess potassium) and hypokalaemia (potassium depletion) can cause abnormalities of cardiac rhythm and even cardiac arrest. Potassium also acts as a part of the buffer system which controls the pH of body fluids. Shifts of hydrogen ions (H +) into and out of cells is compensated by shifts of potassium (K +) in the opposite direction to maintain cation balance.
Potassium balance is similar to sodium balance as it is maintained by renal mechanisms. However, whereas sodium loss or retention is controlled to meet the specific needs of the body, potassium loss is constant. Most potassium is reabsorbed by the proximal tubule but about 10–15% is lost in the urine despite any need changes in the body.
Tubular cell secretion of potassium
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


