Fine Needle Aspiration Biopsy of the Thyroid: Thyroid Lobectomy and Subtotal and Total Thyroidectomy
Herbert Chen
The thyroid gland lies in the central compartment of the neck and is made up of a left and right lobe as well as an isthmus. The isthmus is located just below the cricoid cartilage (Fig. 1). The thyroid normally weighs 14 to 20 g. The arterial supply is from the superior thyroid artery (the first branch of the external carotid) and the inferior thyroid artery from the thyrocervical trunk. The venous drainage of the thyroid comprises the superior thyroid vein, which drains into the internal jugular vein, the middle thyroid vein (only present in 50% of individuals), and the inferior thyroid vein, which drains into the innominate/brachiocephalic veins. The thyroid contains 90% of the body’s iodine. Iodine is oxidized and binds to tyrosine residues in thyroglobulin (colloid). About 1% of thyroid hormone is released every day. The half-lives of the active forms of thyroid hormone are 7 days for T4 and 1 to 3 days for T3.
History
When evaluating a patient presenting with thyroid disease, one should inquire about symptoms of hyperthyroidism or hypothyroidism, risk factors for thyroid cancer, and symptoms due to enlargement of the gland. Symptoms of hyperthyroidism include heat intolerance, weight loss, palpitations, hair loss, and diarrhea. Symptoms of hypothyroid include cold intolerance, fatigue, weight gain, constipation, and hoarseness. Thyroid nodules occur in 4% to 7% of the general population. The vast majority of these nodules (95%) are benign. Risk factors for thyroid cancer include hypothyroidism, a family history of thyroid cancer (especially papillary or medullary thyroid cancer), and a history of head/neck irradiation. Large thyroid nodules or diffusely enlarged glands can cause compressive symptoms involving the esophagus, trachea, and recurrent laryngeal nerve such as dyspnea, stridor, orthopnea, dysphagia, or hoarseness. Recent data suggest that even moderately enlarged thyroid gland/nodules can cause significant swallowing dysfunction that is relieved with thyroidectomy.
Physical Examination
Signs of hyperthyroidism include elevated pulse, tremor, warm skin, and prominent eyes. Signs of hypothyroidism include dry skin, nonpitting edema, and coarse hair. The thyroid gland can be palpated easily by standing behind the patient and locating the cricoid cartilage. The isthmus lies just below the cricoid. Asking the patient to drink water while palpating the neck with both hands will facilitate localization of thyroid mass since the thyroid moves up and down with swallowing. Thyroid nodules are usually quite firm. It is important to also examine the central and lateral neck lymph nodes for lymphadenopathy.
The most useful laboratory test for screening patients with thyroid disease is measurement of thyroid stimulating hormone (TSH), which is suppressed in patients with hyperthyroidism and elevated in patients with hypothyroidism. In patients with a suppressed TSH and symptoms/signs of hyperthyroidism, a thyroid scan is the next step to determine the etiology. Patients with Graves’ disease, which comprise 70% of hyperthyroidism, will have diffuse uptake, while patients with a toxic thyroid nodule will have a single focus of uptake, and those with Plummer’s disease (multiple hot thyroid nodules) will have multiple-foci uptake. In the absence of hyperthyroidism, thyroid scans have limited utility in the workup of thyroid nodules.
The best imaging study for the thyroid is cervical ultrasound. Findings on ultrasound suspicious for thyroid malignancy include microcalcifications, hypoechogenicity, gross local invasion, irregular margins, and regional lymphadenopathy. Ultrasound is critical for guiding fine needle aspiration (FNA), which is the procedure of choice for the evaluation and management of thyroid nodules.
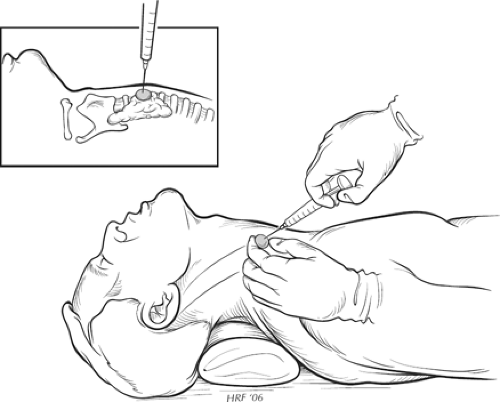 Fig. 1. FNA biopsy diagram with neck extended, nondominant hand on nodule, and needle in dominant hand. |
Fna Biopsy
FNA is a relatively simple and safe procedure, and it is usually performed in the outpatient clinic. The patient is placed in a
supine position with a pillow or towel roll placed behind the shoulder to extend the neck and to bring the thyroid closer to the surface of the skin (Fig. 1). At our institution, the patient often has EMLA cream applied to the skin 20 to 30 minutes prior to the procedure. The skin is then prepped with alcohol, and 1% lidocaine without epinephrine can be injected into the skin for additional local anesthesia. The thyroid mass is localized with ultrasound and immobilized between the fingertips of the nondominant hand. Using the dominant hand, a 23- or 25-gauge 1.5-in. needle with an attached 10-mL syringe is advanced into the lesion, with the clinician noting the consistency of the nodule upon entering. We prefer the nonsuction technique because it results in less trauma and bleeding. If utilizing the suction technique, the syringe is pulled back with the thumb once the lesion has been entered. A syringe-holder aspiration device can facilitate this technique. Once the needle enters the lesion, it is rapidly moved back and forth along a single track for each aspiration until material is seen with the hub of the needle. Suction (if utilized) is released before removal of the needle from the nodule. Firm pressure is applied to the puncture site. Three to six passes are often required to obtain an adequate sample. If a cyst is encountered, the fluid is completely aspirated and sent for cytologic examination. The region of the cyst is then evaluated by ultrasound and any residual solid component reaspirated.
supine position with a pillow or towel roll placed behind the shoulder to extend the neck and to bring the thyroid closer to the surface of the skin (Fig. 1). At our institution, the patient often has EMLA cream applied to the skin 20 to 30 minutes prior to the procedure. The skin is then prepped with alcohol, and 1% lidocaine without epinephrine can be injected into the skin for additional local anesthesia. The thyroid mass is localized with ultrasound and immobilized between the fingertips of the nondominant hand. Using the dominant hand, a 23- or 25-gauge 1.5-in. needle with an attached 10-mL syringe is advanced into the lesion, with the clinician noting the consistency of the nodule upon entering. We prefer the nonsuction technique because it results in less trauma and bleeding. If utilizing the suction technique, the syringe is pulled back with the thumb once the lesion has been entered. A syringe-holder aspiration device can facilitate this technique. Once the needle enters the lesion, it is rapidly moved back and forth along a single track for each aspiration until material is seen with the hub of the needle. Suction (if utilized) is released before removal of the needle from the nodule. Firm pressure is applied to the puncture site. Three to six passes are often required to obtain an adequate sample. If a cyst is encountered, the fluid is completely aspirated and sent for cytologic examination. The region of the cyst is then evaluated by ultrasound and any residual solid component reaspirated.
The needle is then detached from the syringe, and the syringe filled with air, reattached, and the contents expelled onto a glass slide. A second slide is placed on top of the first slide and the material is smeared by pulling the slides in opposing horizontal directions. Slides can be either immediately placed into alcohol or sprayed with fixative for Papanicolaou’s stain or air-dried for Diff-Quik (May-Grunwald-Giemsa) staining. The definition of specimen adequacy varies from institution to institution. Usually at least 6 to 10 clusters of cells on two separate slides are required to make a diagnosis. On-site evaluation by a cytopathologist can significantly reduce the inadequacy rate. Complications from FNA are extremely rare but include bleeding/hematoma (<0.5%), tracheal puncture (rare), nodule infarction (<5%), and tumor seeding (<0.005%).
FNAs are usually classified into four categories:
benign: nodular goiter, Hashimoto’s thyroiditis, subacute thyroiditis, cyst, and colloid nodule;
suspicious/indeterminate: follicular neoplasm, Hurthle cell neoplasm, suspicious but not diagnostic of papillary thyroid cancer;
malignant: papillary thyroid cancer, medullary thyroid cancer, anaplastic thyroid cancer, lymphoma, metastases; and
inadequate/nondiagnostic.
FNAs in the latter category should be repeated as up to 80% may be diagnostic on the repeat attempt.
Malignant Fna
Papillary thyroid cancer. For lesions <1 cm, either thyroid lobectomy or total thyroidectomy is acceptable. For lesions 1 cm or greater, total thyroidectomy is recommended. If abnormal lymph nodes are seen intraoperatively or by ultrasound, level 6 central lymph node dissection is indicated. In patients with enlarged lateral lymph nodes, FNA should be performed on the lymph node and if positive a compartmentalized lymph node dissection often involving levels 2, 3, and 4 lymph nodes should be done.
Medullary thyroid cancer. Total thyroidectomy with bilateral central (level 6) lymph node dissection is the minimal operation. For patients with abnormal lateral lymph nodes by ultrasound or computed tomography (CT), or in patients with calcitonin levels >1000 pg/mL, modified radical neck dissection should be performed. Because of the association of medullary thyroid cancer with multiple endocrine neoplasia type 2 A (MEN 2 A), preoperative evaluation should include plasma and/or 24 urinary metanephrines for pheochromocytoma, CT scans of the neck, chest, and abdomen for staging, calcium and parathyroid hormone testing for hyperparathyroidism, and RET proto-oncogene testing to assess for familial disease.
Thyroid lymphoma and anaplastic thyroid cancer. These types of thyroid malignancies are primarily treated with chemotherapy and/or radiation therapy. Occasionally, thyroidectomy is performed for early anaplastic cancers, and for palliation of airway compromise from thyroid lymphoma.
Suspicious/Indeterminate Fna
Follicular neoplasm. Approximately 80% of follicular neoplasms or lesions are adenoma while 20% are cancer. The presence of capsular and/or vascular invasion on permanent histology distinguishes adenomas from follicular cancers. We generally perform a diagnostic thyroid lobectomy in patients without frozen section. We and others have demonstrated that intraoperative frozen section is misleading and does not provide any additional information >90% of the time. Thus, we wait for permanent histology and if positive for cancer perform a completion thyroidectomy usually within 5 days or after 2 to 3 months from the original thyroid lobectomy. For patients with a follicular neoplasm, we would consider an initial total thyroidectomy for the following: contralateral nodular disease or Hashimoto’s thyroiditis, the patient is already taking thyroid hormone, or patient preference.
Hurthle cell neoplasm. Approximately 70% of Hurthle cell neoplasms or lesions are adenoma while 30% are cancer. Similar to follicular neoplasms, the presence of capsular and/or vascular invasion on permanent histology distinguishes Hurthle cell adenomas from cancers. Likewise, we generally perform a diagnostic thyroid lobectomy in patients without frozen section. If the permanent histology is positive for cancer, we perform a completion thyroidectomy. Many studies have shown that the rate of malignancy for Hurthle cell neoplasms is directly related to the size of the lesion. While cancer is very uncommon with Hurthle cell neoplasms less than 2 cm in size, the rate of cancer exceeds 50% in lesions of a greater size than 4 cm. Thus, we typically recommend a total thyroidectomy for Hurthle cell neoplasms larger than 4 cm. We would also consider a total thyroidectomy in patients with contralateral nodular disease and Hashimoto’s thyroiditis, if the patient was already taking thyroid hormone, or patient preference.
Suspicious, but not diagnostic, of papillary thyroid cancer. An aspirate “suspicious for papillary thyroid cancer” is not the same as an aspirate “diagnostic for papillary thyroid cancer” in regard to likelihood of malignancy and surgical management. The cytologic criteria for papillary thyroid cancer include large monolayer sheets of follicular epithelial cells, enlarged nuclei with powdery chromatin, intranuclear cytoplasmic inclusions and grooves, and papillary structures with or without tall columnar cells. While FNAs diagnostic of papillary thyroid cancer have all of these features, those FNAs that have some but not all of the features of papillary thyroid cancer are read as “suspicious for papillary thyroid cancer.” Multiple reports have shown that FNAs suspicious for papillary thyroid cancer are malignant 50% to 60% of the time. Thus, for a thyroid nodule that is suspicious for papillary thyroid cancer, we would recommend a thyroid lobectomy to
resect the nodule, with intraoperative frozen section analysis. We and others have demonstrated that frozen section is extremely useful with an FNA suspicious for papillary thyroid cancer, as it is accurate 90% of the time. If the frozen section is positive for papillary thyroid cancer, we would perform a total thyroidectomy at that time. If the frozen section is negative, we would terminate the operation having performed only a lobectomy and wait for final histologic evaluation.
resect the nodule, with intraoperative frozen section analysis. We and others have demonstrated that frozen section is extremely useful with an FNA suspicious for papillary thyroid cancer, as it is accurate 90% of the time. If the frozen section is positive for papillary thyroid cancer, we would perform a total thyroidectomy at that time. If the frozen section is negative, we would terminate the operation having performed only a lobectomy and wait for final histologic evaluation.
Benign Fna
The management of a thyroid nodule that is “benign” on FNA is dependent upon the size of the nodule and if the patient has symptoms due to the nodule. Benign nodules generally do not require surgery unless they are causing compressive symptoms (airway compromise, dysphagia, etc.). Data from multiple investigators have shown that thyroidectomy in symptomatic patients can greatly improve quality of life. Thyroidectomy should also be considered in patients with thyroid nodules and a history of head and neck irradiation because of the increased risk of developing thyroid cancer. We generally advocate a total thyroidectomy in patients with thyroid nodules and a history of head and neck irradiation, irrespective of biopsy findings.
Nodule greater or equal to 4 cm in size. While FNA is extremely accurate in delineating most benign from malignant thyroid nodules, several studies have shown that FNA is less reliable with thyroid nodules ≥4 cm. McCoy and colleagues noted that preoperative FNA results in patients with thyroid nodules ≥4 cm were read incorrectly as benign in 13% of patients with cancer; when multifocal micropapillary carcinoma was included, this false-negative rate for preoperative FNA reached 16%. In a study from our group, FNA results reported as benign turned out to be either neoplastic (22/52) or malignant (4/52) on final pathology. Among patients with nondiagnostic FNAs, the risk of malignancy was 27%. We concluded that in patients with thyroid nodules ≥4 cm, FNA results are highly inaccurate, misclassifying half of all patients with reportedly benign lesions on FNA. Furthermore, those patients with a nondiagnostic FNA display a very high risk of differentiated thyroid carcinoma. Therefore, we recommend that diagnostic lobectomy, at a minimum, be performed in patients with thyroid nodules ≥4 cm regardless of FNA cytology. In patients who are asymptomatic and have nodules <4 cm, management usually consists of clinical follow-up with a repeat thyroid ultrasound in 6 months. If the nodule increases in size or becomes worrisome in ultrasound appearance during follow-up, repeat FNA is warranted. Our practice has been to follow small, asymptomatic thyroid nodules at 6, 18, and 42 months.
Hyperthyroidism. Kocher developed subtotal thyroidectomy as the treatment for hyperthyroidism from Graves’ disease, which then became the routine form of therapy for the disease. After the advent of radioactive iodine therapy in the 1930s, surgery became less commonly performed as the primary treatment. There are still a number of important indications for surgical treatment of hyperthyroidism such as age, sex, pregnancy, and lactation, the presence of a thyroid nodule or large goiter (Plummer’s), and patient preference. These factors may guide clinicians to offer surgery as a first-line treatment. For patients with Graves’ disease or Plummer’s disease, total thyroidectomy is the operation of choice and has largely replaced subtotal thyroidectomy, which is associated with a much high recurrence rate and similar morbidity. For patients with hyperthyroidism due to a single hot nodule, unilateral thyroid lobectomy is the best operation.
Informed consent. As with any operation, the surgeon should have a thorough discussion with the patient about the indications, alternate treatment options, and potential complications of thyroidectomy. Complications for thyroid lobectomy include injury to the recurrent laryngeal nerve, resulting in a hoarse voice, and external branch of the superior laryngeal nerve, leading to an inability to reach the high octaves when singing. I typically quote a 5% to 10% rate of temporary/transient hoarseness and a 1% to 2% rate of long-term voice complications.
The parathyroid glands could also be inadvertently injured. This does not pose a problem with a thyroid lobectomy (since the contralateral two parathyroids would be sufficient), but increases the risk of hypoparathyroidism should future thyroid or parathyroid surgery be required since the remaining parathyroid glands would be at risk. In patients undergoing a total thyroidectomy, a 10% to 20% rate of transient hypocalcemia and a 1% to 2% rate of permanent hypocalcemia required calcitriol supplementation. We and others have shown that hypoparathyroidism for most patients usually resolves within 1 week.
Postoperative bleeding and subsequent hematoma formation is a potential life-threatening complication that must be carefully monitored during the postoperative period. This occurs in <1% of cases. Wound infections are uncommon. The most common wound complication is seroma formation that usually resolves spontaneously.
Patients should be aware that after total or near-total thyroidectomy they will be required to take lifelong thyroid hormone replacement. In patients undergoing thyroid lobectomy, the vast majority (>85%) will not require thyroid hormone. However, we and others have described risk factors that increase the chance of hypothyroidism after thyroid lobectomy, which include a high-normal TSH, Hashimoto’s thyroiditis, and a low T4 level. When patients are stratified into three groups based on their preoperative TSH measurement (≤1.5, 1.51 to 2.5, and ≥2.51 μIU/mL), the rate of hypothyroidism after thyroid lobectomy increases significantly at each level (13.5%, 20.5%, and 41.3%, respectively). Thus, preoperative TSH levels can be used to predict the likelihood of postoperative hypothyroidism.
Preoperative testing. In all patients undergoing thyroid surgery, it is our practice to check the preoperative calcium and parathyroid hormone level to rule out hyperparathyroidism, and to obtain a baseline value for comparison. In patients who are diagnosed with hyperparathyroidism, we perform parathyroidectomy at the time of thyroidectomy. If the patient has a hoarse voice preoperatively or if has had a previous operation that placed the vagus or recurrent laryngeal nerve at risk, he or she should have direct or indirect laryngoscopy preoperatively to assess the status of the recurrent laryngeal nerves. A paralyzed nerve may alter operative plans and should definitely be discussed when obtaining informed consent. A procedure planned on the side contralateral to a nerve injury risks bilateral nerve injury and the need for tracheostomy.
Hyperthyroidism. It is important to restore a patient with hyperthyroidism to a euthyroid state prior to surgery to avoid the potential of precipitating a thyroid storm during surgery. This can be accomplished within 6 weeks using an antithyroid drug such as PTU 100 to 300 mg three times daily or methimazole 10 to 30 mg three times a day. Methimazole is usually changed to a single daily dose once a patient is euthyroid since it has a longer duration of action compared with PTU. Propranolol in doses of 40 to 120 mg four times a day is often added to control symptoms of tachycardia, tremor,
heat intolerance, and anxiety. Propranolol is usually initiated simultaneously with the antithyroid drug, but is continued about 1 week after surgery since the half-life of T4 is 7 days. We administer a saturated solution of potassium iodide (SSKI) in a dose of 1 drop two to three times daily or Lugol’s solution in a dose of 5 to 10 drops two to three times daily about 10 to 14 days prior to surgery to decrease the vascularity of the thyroid gland and facilitate surgical resection. These agents are discontinued immediately after surgery. When a patient requires urgent surgery, rapid preparation can be accomplished within 7 days using a combination of a corticosteroid (dexamethasone 2 mg every 6 hours), propranolol 40 mg every 8 hours, and sodium iopanoate (500 mg every 6 hours). Since both propranolol and sodium iopanoate have a rapid onset of action, it is worth starting these two agents even in truly emergent cases.
heat intolerance, and anxiety. Propranolol is usually initiated simultaneously with the antithyroid drug, but is continued about 1 week after surgery since the half-life of T4 is 7 days. We administer a saturated solution of potassium iodide (SSKI) in a dose of 1 drop two to three times daily or Lugol’s solution in a dose of 5 to 10 drops two to three times daily about 10 to 14 days prior to surgery to decrease the vascularity of the thyroid gland and facilitate surgical resection. These agents are discontinued immediately after surgery. When a patient requires urgent surgery, rapid preparation can be accomplished within 7 days using a combination of a corticosteroid (dexamethasone 2 mg every 6 hours), propranolol 40 mg every 8 hours, and sodium iopanoate (500 mg every 6 hours). Since both propranolol and sodium iopanoate have a rapid onset of action, it is worth starting these two agents even in truly emergent cases.
Perioperative considerations. Patients should urinate immediately preoperatively so that there is no need for a Foley catheter. As thyroidectomy is classified as a “clean” operative procedure, prophylactic antibiotics are not required unless the patient has a special medical condition warranting their administration. Compression stockings and sequential compression devices are used selectively for deep vein thrombosis (DVT) prophylaxis. Heparin should be used very selectively since recent data suggest that the risk of postoperative neck hematoma overweighs the incidence of DVT in patients undergoing thyroidectomy.
Thyroid lobectomy. Following the induction of general anesthesia, the patient remains in the supine position, arms straight and tucked at their sides, and generous padding is placed at the elbows to prevent nerve injury. The patient’s neck is midline and extended. This neck extension is performed with extreme caution and with the assistance of the anesthesia team to ensure that the endotracheal tube is secured and that the cervical spine is not overextended or suspended. Preoperative assessment should include asking the patient to fully extend his or her neck, so that the person positioning the patient knows the level of natural neck extension. Hyperextension of the neck may lead to increased postoperative pain and a slight risk of spinal cord damage. Perfect alignment of the head and body must be ensured to prevent erroneous placement of the cervical incision. Appropriate positioning ensures that the isthmus of the thyroid overlies the second and third tracheal rings just caudal to the cricoid cartilage (Fig. 2). A deflated IV bag is placed under the patient’s shoulders to extend the neck and support the shoulders and lower cervical spine. The bag is then inflated to produce the appropriate amount of neck extension. The head should be well supported using a head ring. A headlight facilitates lighting and exposure through the limited incisions. During the operation, the table is placed in a beach-chair position to decrease the cervical venous pressure.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


