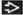Fibroadenoma
Key Facts
Terminology
Fibroadenoma (FA)
Most common benign solid breast neoplasm
Well-circumscribed lesion
Proliferation of epithelial and stromal elements
Etiology/Pathogenesis
FAs are due to proliferation of lobular stroma and may be polyclonal or monoclonal
Clinical Issues
Typically occur in younger patients between ages 20-35
Painless, slowly growing, mobile, well-defined mass
Most FAs can be diagnosed by core needle biopsy and followed radiographically
Image Findings
Circumscribed or lobulated mass
Calcifications may be present, particularly in older women
Microscopic Pathology
Admixed glands and stromal elements
Top Differential Diagnoses
Phyllodes tumor
Hamartoma (fibroadenolipoma)
Fibroadenomatoid changes
Fibrous tumor
Pseudoangiomatous stromal hyperplasia
Tubular adenoma
TERMINOLOGY
Abbreviations
Fibroadenoma (FA)
Synonyms
Adenofibroma
Definitions
Biphasic fibroepithelial tumor consisting of intralobular stromal cells and associated epithelial cells
ETIOLOGY/PATHOGENESIS
Abnormal Growth of Intralobular Stromal Cells
Normal breast development
During embryological development, stroma differentiates 1st and induces downgrowth of cells from epidermis to form ductal system
This synergistic relationship between epithelial and stromal cells persists in duct/lobular unit
Increased growth of stromal cells is accompanied by corresponding hyperplasia of epithelial cells
Several possible etiologies for abnormal growth of intralobular stromal cells
Hormonal stimulation
Most FAs are polyclonal hyperplasias of lobular stroma
Some stromal cells have estrogen receptor β &/or progesterone receptors
FAs occur most commonly in young premenopausal women
FAs can grow during pregnancy
If rapid growth occurs, lesion may infarct
May be mistaken for malignancy
Iatrogenic
Cyclosporine in kidney transplant recipients is associated with increase in FAs
Attributed to possible similarity to prolactin
Can regress when patient is switched to different medication
Genetic/hereditary
More common in African-American women
Myxoid FAs occur in Carney complex
Myxomas (cardiac, cutaneous, breast), primary pigmented nodular adrenocortical disease, large cell calcifying Sertoli cell tumors, multiple thyroid lesions, growth hormone-producing pituitary adenoma, other tumors
Pigmented skin lesions (lentigos, blue nevi, café au lait spots), typically involving vermillion border of lip and intercanthal portion of eye
Type 1 (CNC1): PRKAR1A (17q23-24)
Type 2 (CNC2): Locus at 2p16
30% of patients do not have an identified germline mutation
Neoplastic
Some FAs are monoclonal stromal tumors
Clonal genetic changes may be present in stromal cells
Associated epithelial cells are usually polyclonal
In general, FAs have few genetic changes
Some have gain of 1q similar to phyllodes tumors
As stromal proliferation becomes more pronounced and autonomous, spectrum of changes seen in FAs overlaps with low-grade phyllodes tumors
Some phyllodes tumors likely arise from FAs
CLINICAL ISSUES
Epidemiology
Presentation
Most commonly presents as painless, slowly growing, mobile, well-defined, palpable nodule in a young woman
In older women, may be detected as mammographic circumscribed density or calcifications
Natural History
May regress in size with age
Stroma typically undergoes hyalinization, which can serve as substrate for calcifications
Treatment
Surgical approaches
Most FAs can be diagnosed by core needle biopsy and followed radiographically
Surgery to remove a FA may be indicated for large lesions, if patient requests removal, or for rare lesions that continue to grow in size
Prognosis
FAs are benign
Only clinical importance is in distinguishing FAs from malignancies
For some women, FAs may be excised due to cosmetic issues if lesion is large
FAs are classified as proliferative breast disease without atypia
Relative risk increased 1.5-2x; absolute lifetime risk of breast cancer is 5-7%
Risk is to both breasts
In 1 study, only women with complex FAs had increased risk
Core Needle Biopsy
Diagnosis of FA can usually be made on core biopsy
Targeted lesion is usually a circumscribed mass or cluster of calcifications
Diagnosis of FA does not correlate well with an irregular mass
Some fibroepithelial lesions on core needle biopsy are difficult to classify as FA or phyllodes tumor
Stroma may show increased cellularity
Mitoses may be present
Mitoses > 2 per 10 HPFs favors phyllodes tumor; lower scores do not discriminate between these lesions
Ki-67 > 5% favors phyllodes tumor; lower scores do not discriminate between these lesions
Because FAs can grow during pregnancy, increased proliferation may be present at this time
Focal stromal overgrowth may be seen
Infiltration of adjacent stroma may be difficult to evaluate due to fragmentation of cores
Certain clinical features increase likelihood that lesion is a phyllodes tumor
Large size
Increase in size
History of prior phyllodes tumor
If a definitive diagnosis cannot be made, lesion should be classified as a “fibroepithelial tumor”
This type of lesion is best classified after complete surgical excision
IMAGE FINDINGS
Mammographic Findings
Circumscribed or lobulated mass
May be ill defined if obscured by dense stroma or if fibroadenomatoid changes are present
Calcifications may be present, particularly in older women, and will appear as a cluster
Calcifications may be coarse (large “popcorn” calcifications) or numerous and small
May mimic calcifications seen in DCIS due to clustering
Ultrasonographic Findings
Circumscribed or lobulated mass
MR Findings
Smooth bordered mass; may have nonenhancing internal septations
Enhancement is generally slower than that seen with carcinomas
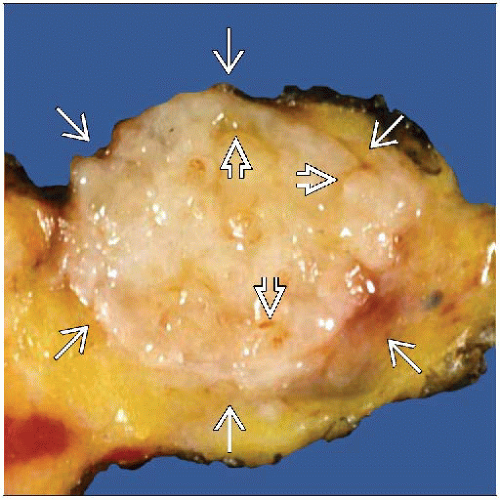
MACROSCOPIC FEATURES
General Features
Circumscribed, white, firm but not hard (rubbery), palpable mass
Mass usually bulges above normal breast tissue; slit-like spaces may be present
Carcinomas usually do not bulge; typically have flat surface
Large FAs may have a leaf-like appearance due to areas of stromal growth separated by epithelial-lined clefts
Multiple synchronous FAs can be present
Size
Majority < 3 cm but can be very large
MICROSCOPIC PATHOLOGY
Histologic Features
Biphasic growth pattern
Admixed glandular and stromal elements
Variable morphologic appearance, depends on relative amount and configuration of each element
Regular and symmetric distribution of epithelial and fibrous elements should be present
FAs typically have well-circumscribed pushing borders and do not infiltrate adjacent parenchyma
Can be difficult to distinguish infiltration of stroma from fibroadenomatoid changes in adjacent stroma
Infarction can occur during pregnancy
Resulting necrosis should not be interpreted as indicating malignancy
Stromal component
Typically loose and may have myxoid appearance
Myxoid stroma is more typical in younger individuals
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree