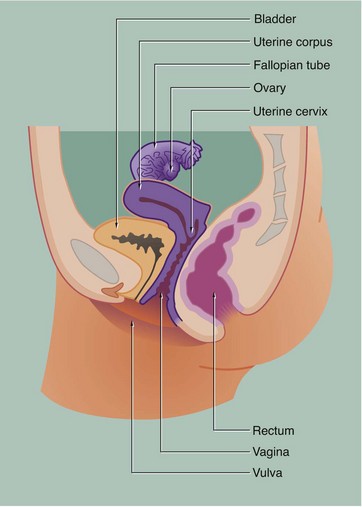
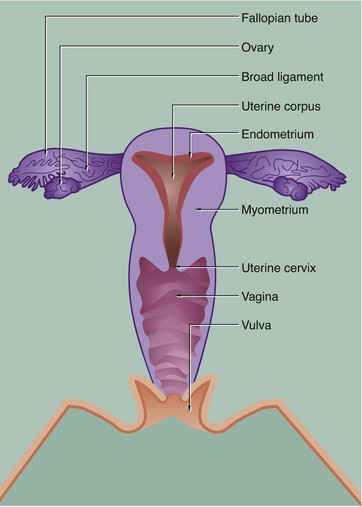
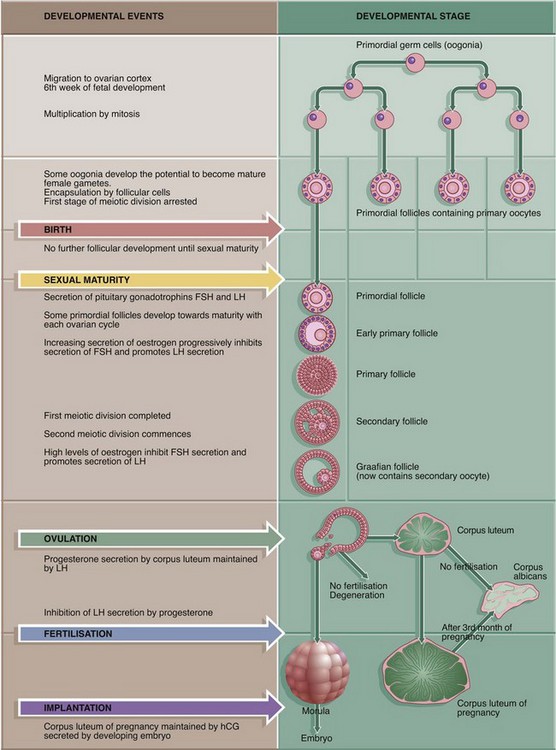
19 The female reproductive system has six major functions: • Production of female gametes, the ova, by the process of oogenesis • Reception of male gametes, the spermatozoa • Provision of a suitable environment for the fertilisation of ova by spermatozoa • Provision of an environment for the development of the fetus • Expulsion of the developed fetus to the external environment • The ovaries, which are the site of oogenesis, are paired organs lying on either side of the uterus adjacent to the lateral wall of the pelvis. In sexually mature mammals, ova are released by the process of ovulation in a cyclical manner, either seasonally or at regular intervals throughout the year. This cycle is suspended during pregnancy. The ovaries are also endocrine organs, producing the hormones oestrogen and progesterone. Both ovulation and ovarian hormone production are controlled by the cyclical release from the anterior pituitary of the gonadotrophic hormones luteinising hormone (LH) and follicle stimulating hormone (FSH). Oestrogen and progesterone in turn regulate LH and FSH production by feedback mechanisms. Thus, ovulation is coordinated with preparation of the uterus to receive the fertilised ovum. • The genital tract extends from near the ovaries to an opening at the external surface and provides an environment for reception of male gametes, fertilisation of ova, development of the fetus and expulsion of the fetus at birth. The genital tract begins with a pair of Fallopian tubes, also called oviducts or uterine tubes, which conduct ova from the ovaries to the uterus where fetal development occurs. Fertilisation of ova by spermatozoa occurs within the Fallopian tubes. The uterus is a muscular organ, the mucosal lining of which undergoes cyclical proliferation under the influence of ovarian hormones. This provides a suitable environment for implantation of the fertilised ovum and subsequent development of the placenta. This is the means by which the developing fetus is nourished throughout gestation. At birth (parturition), strong contractions of the muscular uterine wall expel the fetus through the lower part of the uterus, the uterine cervix, into the birth canal or vagina. The vagina is an expansile muscular tube specialised for the reception of the penis during coitus and for the passage of the fetus to the external environment. At the external opening of the vagina there are thick folds of skin, the labia which, along with the clitoris, constitute the vulva. • The breasts are highly modified apocrine sweat glands which, in the female, develop at puberty and regress at menopause. During pregnancy, the secretory components expand greatly in size and number in preparation for milk production (lactation). The general anatomy of the female genital tract is illustrated in Figs 19.1 and 19.2. FIG. 19.3 Ovary (illustrations (a) and (b) opposite) During early fetal development, primordial germ cells called oogonia migrate into the ovarian cortex where they multiply by mitosis. By the fourth and fifth months of human fetal development, some oogonia enlarge and assume the potential for development into mature gametes. At this stage, they are called primary oocytes and commence the first stage of meiotic division (see Ch. 2). By the seventh month of fetal development, a single layer of flattened follicular cells surrounds the primary oocytes to form primordial follicles, of which there are approximately 500 000 in the human ovary at birth. This encapsulation arrests the first meiotic division and no further development of primordial follicles then occurs until after the female reaches sexual maturity (puberty). The process of meiotic division is only completed during follicular maturation, leading up to ovulation and fertilisation. Thus, all the female germ cells are present at birth, but the process of meiotic division is only completed some 15 to 50 years later! In contrast, in males, meiotic division of germ cells commences only after sexual maturity and formation and maturation of spermatozoa are accomplished within about 70 days (see Ch. 18). Female germ cells may undergo degeneration (atresia) at any stage of follicular maturation. FIG. 19.4 Ovarian cortex FIG. 19.5 Secondary follicle FIG. 19.6 Graafian follicle FIG. 19.8 Corpus luteum of menstruation FIG. 19.9 Corpus luteum of pregnancy FIG. 19.10 Atretic follicles FIG. 19.11 Corpus albicans FIG. 19.12 Fetal ovary FIG. 19.13 Fallopian tubes FIG. 19.14 Fallopian tube
Female reproductive system
Introduction
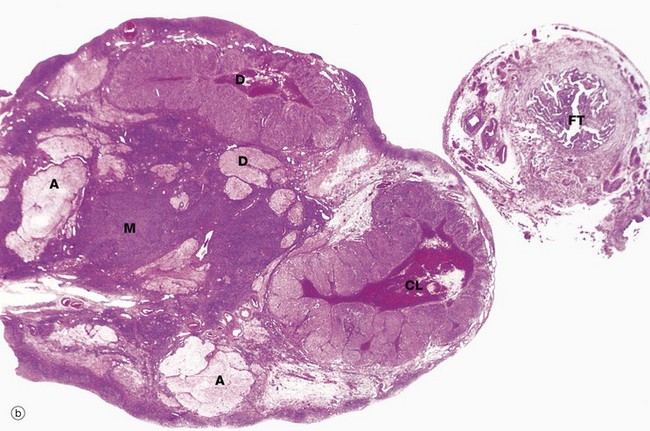
(a) Monkey, Azan (LP) (b) Human, H&E (LP)
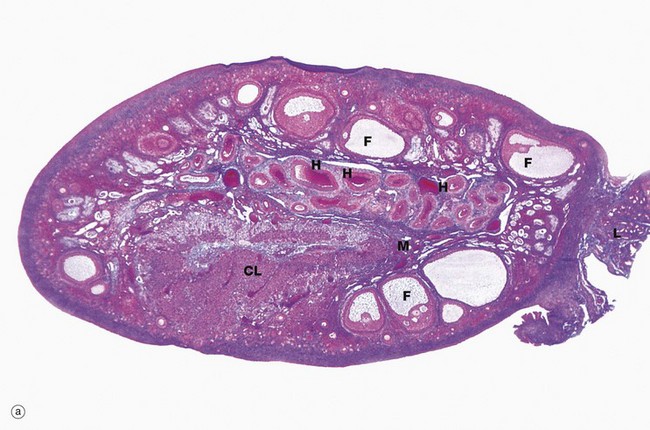
The ovaries of all mammals have a similar basic structure. There are, however, considerable variations in accordance with species differences in the ovarian cycle and the stage in the cycle at which the ovary is examined. These micrographs compare the ovarian appearance of the monkey with that of the human.
The ovaries, which are some 3 to 5 cm long in humans, have a flattened ovoid shape. The body of the ovary consists of spindle-shaped cells, fine collagen fibres and ground substance that together constitute the ovarian stroma. The stromal cells resemble fibroblasts, but some contain lipid droplets. Bundles of smooth muscle cells are also scattered throughout the stroma. In the peripheral zone of the stroma, known as the cortex, there are numerous follicles that contain female gametes in various stages of development. In addition, there may also be post-ovulatory follicles of various kinds, namely corpora lutea (responsible for oestrogen and progesterone production, see Fig. 19.8), degenerate and former corpora lutea (corpora albicantes, see Fig. 19.11) and degenerate (atretic) follicles (see Fig. 19.10).
The superficial cortex is more fibrous than the deep cortex and is often called the tunica albuginea. However, unlike the testis, this is not an anatomically distinct capsule. On the surface of the ovary is an epithelial covering, misleadingly called germinal epithelium, which is a continuation of the peritoneum.
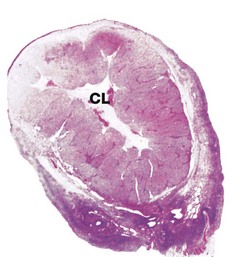
In the monkey ovary, numerous follicles F are seen in various sizes and states of development. In contrast, developing follicles are difficult to see in the human ovary (b) at this magnification. An active corpus luteum CL and several degenerating corpora lutea D and corpora albicantes A dominate this human ovary.
The central zone of the ovarian stroma, the medulla M, is highly vascular and contains hilus cells, which are morphologically very similar to Leydig cells of the testis. The ovarian artery (a branch of the aorta) and ovarian branches of the uterine artery form anastomoses in the mesovarium and the broad ligament L. From this arterial plexus, approximately 10 coiled arteries, the helicine arteries H enter the hilum of the ovary, best seen in micrograph (a). Smaller branches form a plexus at the corticomedullary junction, giving rise to straight cortical arterioles that radiate into the cortex. Here, they branch and anastomose to form vascular arcades, giving rise to a rich network of capillaries around the follicles. Venous drainage follows the course of the arterial system, the medullary veins being large and tortuous. Lymphatics arise in the perifollicular stroma, draining to larger vessels which coil around the medullary veins. Innervation of the ovary is by sympathetic fibres that not only supply blood vessels but also terminate on smooth muscle cells in the stroma around the follicles, possibly playing some part in follicular maturation and ovulation. In micrograph (b), the nearby Fallopian tube FT is included in the plane of section.
Follicular Development
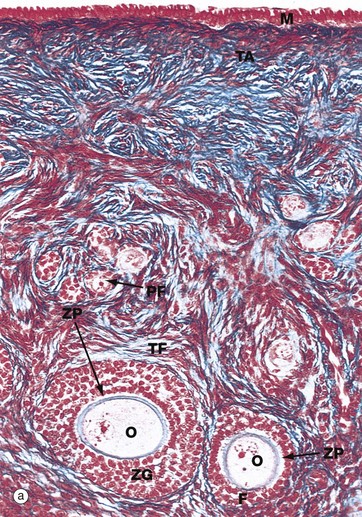
(a) Monkey, Azan (MP) (b) Human, H&E (MP)
Micrograph (a), taken from a monkey, shows the typical appearance of follicles in the ovarian cortex and illustrates several stages in early follicular development.
In the mature ovary, undeveloped follicles exist as primordial follicles PF which are composed of a primary oocyte surrounded by a single layer of flattened follicular cells. The primary oocyte has a large nucleus with dispersed finely granular chromatin, a prominent nucleolus and little cytoplasm.
At the lower right of the field, a primordial follicle has been stimulated, increasing in size to form a primary follicle. Its oocyte O has greatly enlarged and the follicular cells F have multiplied by mitosis and become cuboidal in shape. They are now known as granulosa cells. A thick homogeneous layer of glycoprotein and acid proteoglycans, the zona pellucida ZP, develops between the oocyte and the follicular cells. Both cell types probably contribute to its formation.
With further follicular development, as seen in the large follicle at lower left, the surrounding stromal cells begin to form an organised layer around the follicle called the theca folliculi TF, separated from the granulosa cells by a basement membrane. Theca cells are derived from the fibroblast-like cells of the ovarian stroma. The primary follicle continues to enlarge and the granulosa cells continue to proliferate, forming a layer several cells thick called the zona granulosa ZG.
Note also in this micrograph the fibrous tunica albuginea TA and the single layer of cuboidal or columnar mesothelial cells M on the surface of the ovary. In humans, this mesothelial layer is low cuboidal rather than columnar. This layer is continuous with the mesothelial lining of the peritoneal cavity and was formerly known as the germinal epithelium, based upon the mistaken belief that these cells were the origin of the female germ cells.
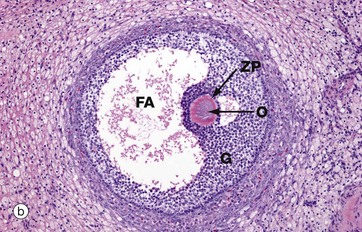
Micrograph (b) illustrates a section of human ovary, showing the next stage in follicular maturation. Fluid-filled spaces develop between the granulosa cells G, and these begin to coalesce to form the follicular antrum FA. This is now known as a secondary follicle. The zona pellucida ZP is well seen in this micrograph, but the nucleus of the oocyte O does not lie in this plane of section. Details of the secondary follicle are illustrated at higher magnification in Fig. 19.5.
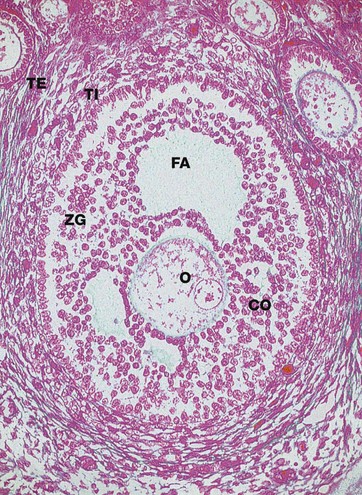
Azan (MP)
Primary follicles continue to develop to form secondary follicles and acquire the features seen in this micrograph. By now they are usually situated deeper in the ovarian cortex.
The zona granulosa ZG continues to proliferate, and small fluid-filled spaces appear within it. These fuse to form the follicular antrum FA, in which follicular fluid accumulates. At this stage, the oocyte O has almost reached its full size and becomes situated eccentrically in a thickened area of the granulosa called the cumulus oophorus CO.
At the periphery of the follicle, the theca folliculi has developed two layers, the theca interna TI, comprising several layers of rounded cells, and the less well-defined theca externa TE, consisting of spindle-shaped cells that merge with the surrounding stroma.
The cells of the theca interna have the features of typical steroid-secreting cells (see Fig. 17.16) and produce oestrogen precursors (e.g. androstenedione), oestrogen and, in the preovulatory stage, progesterone. In the ovary, these steroid-secreting cells are often described as luteinised. Follicular hormones promote proliferation of the endometrium in readiness for the implantation of a fertilised ovum.
The theca externa is composed of flattened stromal cells and has no endocrine function. The granulosa cells also produce hormones from the stage of antral formation onwards. Oestrogen is produced from precursors secreted by the theca interna, as well as small amounts of intrafollicular FSH and, at ovulation, the FSH inhibitor inhibin F.
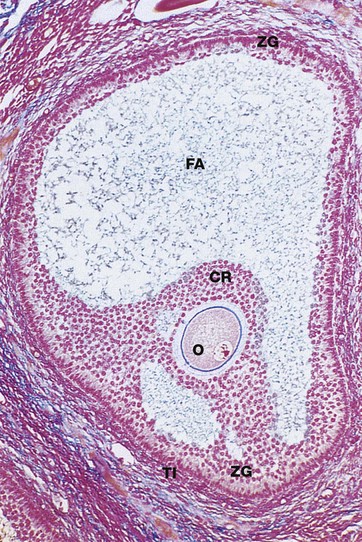
Azan (LP)
Approaching maturity, further growth of the oocyte ceases and the first meiotic division is completed just before ovulation. At this stage, the oocyte becomes known as the secondary oocyte and commences the second meiotic division. The first polar body (see Ch. 2), containing very little cytoplasm, remains inconspicuously within the zona pellucida. The follicular antrum FA enlarges markedly and the zona granulosa ZG now forms a layer of even thickness around the periphery of the follicle. The cumulus oophorus diminishes, leaving the oocyte O surrounded by a layer several cells thick, the corona radiata CR, which remains attached to the zona granulosa by thin bridges of cells. Before ovulation, these bridges break down and the oocyte, surrounded by the corona radiata, floats free inside the follicle.
Note the surrounding theca interna TI, consisting of plump luteinised cells. By this stage, the follicle has reached between 1.5 and 2.5 cm in diameter and bulges under the ovarian surface. The overlying surface epithelial cells are flattened and atrophic and the thin intervening stroma becomes degenerate and avascular.
At ovulation, the mature follicle ruptures and the ovum, made up of the secondary oocyte, zona pellucida and corona radiata, is expelled into the peritoneal cavity near the entrance to the Fallopian tube. The second meiotic division of the oocyte is not completed until after penetration of the ovum by a spermatozoon.
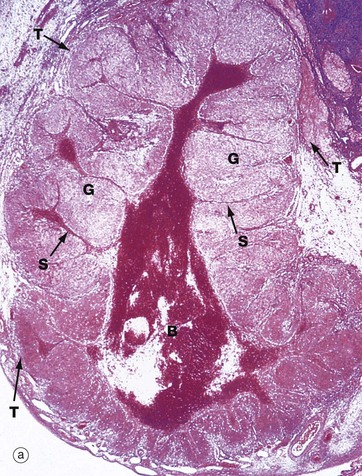
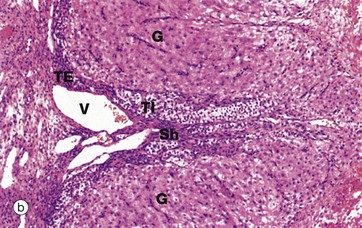
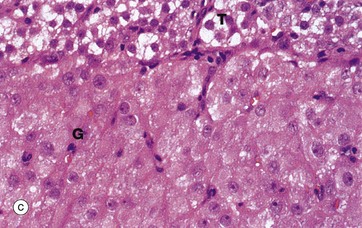
(a) H&E (LP) (b) H&E (MP) (c) H&E (HP)
Following ovulation, the ruptured follicle collapses and fills with a blood clot to form the corpus luteum of menstruation, which has a brief career as an endocrine organ.
The corpus luteum of menstruation is about the same size as the antecedent ovulatory follicle (i.e. 1.5 to 2.5 cm). Under the influence of luteinising hormone (LH) secreted by the anterior pituitary, granulosa cells increase greatly in size and begin secretion of progesterone. The granulosa cells acquire the characteristics of steroid-secreting cells and are now called granulosa lutein cells. Progesterone promotes the changes in the endometrium that make it ready for implantation of the embryo should fertilisation occur (see Figs 19.15 to 19.19). Thus the cycles of production of oocytes and the preparation of the endometrium (the menstrual cycle) are coordinated by the same set of hormones.
The cells of the theca interna also increase somewhat in size and acquire similar cytoplasmic features to the luteinised granulosa cells. Although interrupted by ovulation, these cells (as well as the granulosa cells) continue to secrete oestrogens which are necessary to maintain the thickened uterine mucosa. These cells become known as theca lutein cells.
The basement membrane between the zona granulosa and the theca interna breaks down and these layers are invaded by capillaries and larger vessels from the theca externa to form a rich vascular network, characteristic of endocrine glands.
Progesterone production by the corpus luteum is dependent on LH from the anterior pituitary, but rising progesterone levels inhibit LH production. Without the continuing stimulus of LH, the corpus luteum cannot be maintained and, 12 to 14 days after ovulation, it regresses, ultimately forming a functionless corpus albicans (see Fig. 19.11). Once the corpus luteum regresses, secretion of both oestrogen and progesterone ceases. Without these hormones, the endometrial lining of the uterus collapses, resulting in the onset of menstruation.
Micrograph (a) shows a corpus luteum of menstruation. In the centre, the remnant of the post-ovulatory blood clot B is seen, surrounded by a broad zone of granulosa lutein cells G, penetrated by septa S containing the larger blood vessels. Peripherally, a thin zone of theca lutein cells T can be seen. Externally, the corpus luteum is bounded by a zone of condensed stromal tissue, representing the theca externa of the antecedent Graafian follicle.
Micrograph (b) shows the margin of a corpus luteum at intermediate magnification. Most of the field is occupied by granulosa lutein cells G, large polygonal cells with abundant pale eosinophilic (pink-stained) cytoplasm and round nuclei. The cytoplasm contains plentiful smooth endoplasmic reticulum, abundant mitochondria, lipid droplets and some lipofuscin, giving the corpus luteum a yellow colour macroscopically.
At the periphery, there are theca cells which also extend in a finger-like extension, forming a sheath Sh around blood vessels V. The theca externa cells TE have darker-stained cytoplasm while the luteinised theca interna cells TI have pale cytoplasm due to their content of lipid droplets.
At high magnification in micrograph (c), granulosa lutein cells G may be compared with theca lutein cells T. The eosinophilic cytoplasm of the granulosa lutein cells contains numerous small lipid droplets which give rise to the vacuolated appearance seen in this preparation. Their larger spherical nuclei contain one or two prominent nucleoli. Theca lutein cells are smaller, with a more densely staining cytoplasm but with larger lipid vacuoles. Their ovoid nucleus has a single large nucleolus. The ultrastructure of the endocrine cells of the corpus luteum is characteristic of all steroid secretory cells (see Fig. 17.16).
As previously described, the granulosa lutein cells secrete progesterone (and a small amount of oestrogen) and the theca lutein cells secrete oestrogen precursors which are converted to oestrogen by the granulosa cells.
H&E (LP)
Implantation of a fertilised ovum in the uterine wall interrupts the integrated ovarian and menstrual cycles. After implantation, a hormone called human chorionic gonadotrophin (hCG) is secreted into the maternal circulation by the developing placenta; hCG has an analogous function to LH and maintains the function of the corpus luteum in secreting oestrogen and progesterone until about the 9th week of pregnancy. After this time, the corpus luteum of pregnancy slowly regresses to form a functionless corpus albicans and the placenta takes over the major role of oestrogen and progesterone secretion until parturition.
This micrograph shows a human ovary during the first trimester of pregnancy. The corpus luteum CL is greatly enlarged and by now occupies most of the ovary. The organisation of the corpus luteum of pregnancy is similar to that of menstruation, but there are some histological changes that are almost specific for the corpus luteum of pregnancy. In particular, the granulosa lutein cells contain hyaline, eosinophilic inclusion bodies that tend to enlarge and then calcify as the pregnancy progresses.
(a) H&E (LP) (b) H&E (MP)
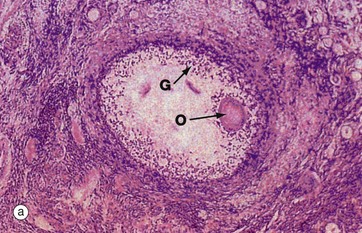
The process of follicular atresia (degeneration) may occur at any stage in the development of the ovum. By the sixth month of development, the fetal ovary contains several million primordial follicles yet, by the time of birth, only about half a million remain. Atresia continues until puberty and thereafter through the reproductive years. In addition, with each ovarian cycle approximately 20 follicles begin to mature, usually all but one becoming atretic at some stage before complete maturity.
The histological appearance of atretic follicles varies enormously, depending on the stage of development reached and the progress of atresia. The atretic follicle seen in micrograph (a) is a secondary follicle in early atresia. The oocyte O has degenerated and the granulosa cells G have begun to disaggregate.
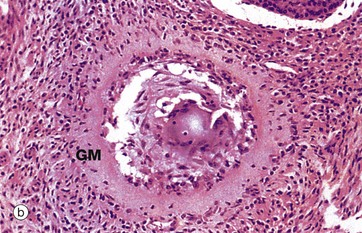
Advanced atresia, as seen in micrograph (b), is characterised by gross thickening of the basement membrane between the granulosa cells and the theca interna, forming the so-called glassy membrane GM. Atretic follicles are ultimately replaced completely by collagenous tissue known as the corpus fibrosum. Most corpora fibrosa eventually disappear completely. In the postmenopausal woman, primordial follicles are absent, and the cortex consists of stroma and corpora albicantes only, with no developing follicles. The postmenopausal ovary is smaller than that in premenopausal women.
H&E (LP)
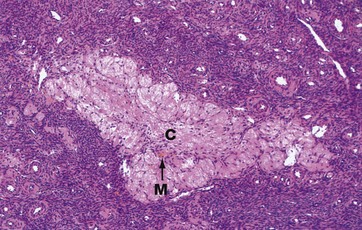
The corpus albicans C is the inactive fibrous tissue mass that forms following the involution of a corpus luteum. The secretory cells of the degenerate corpus luteum undergo autolysis and are phagocytosed by macrophages M, a few of which, containing cytoplasmic haemosiderin pigment, can be seen here. The vascular supporting tissue regresses to form a relatively acellular collagenous scar containing a few fibroblasts. In the human ovary, corpora albicantes are a dominant feature, increasing in number with age and often appearing to occupy almost the whole ovarian stroma. Most regress completely leaving no trace, otherwise the postmenopausal ovary would contain approximately 500 corpora albicantes.
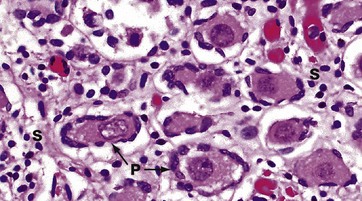
H&E (HP)
In this micrograph of ovary from a term fetus, the ovarian cortex is seen to be packed with primordial follicles P. The surrounding stroma S is much more delicate than in an adult woman. These ova are arrested in the first meiotic division and remain so until the onset of puberty signals the waves of maturation of follicles that occur with each cycle in the reproductive years.
The Genital Tract
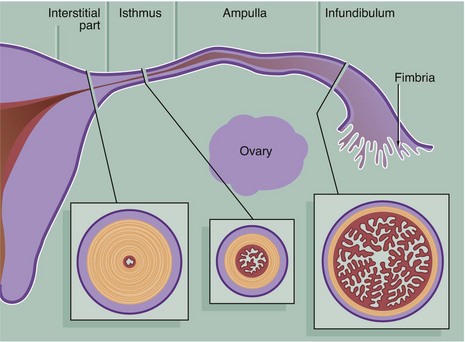
The Fallopian tubes (also called uterine tubes or oviducts) carry ova from the surface of the ovaries to the uterine cavity and are also the site of fertilisation by spermatozoa. The Fallopian tube is shaped like an elongated funnel and is divided anatomically into four parts as shown in the diagram.
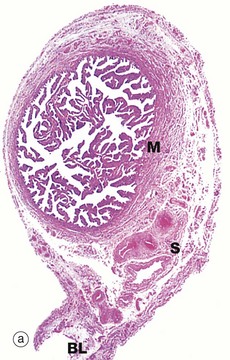
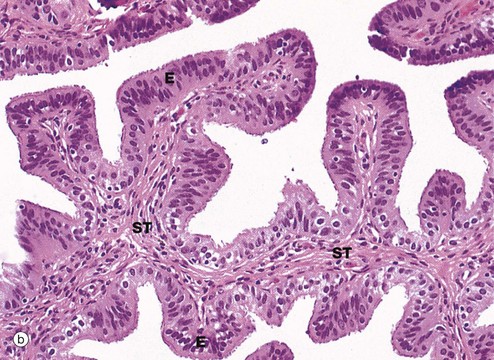
(a) H&E (LP) (b) H&E (MP) (c) H&E (HP) (d) Azan (HP)
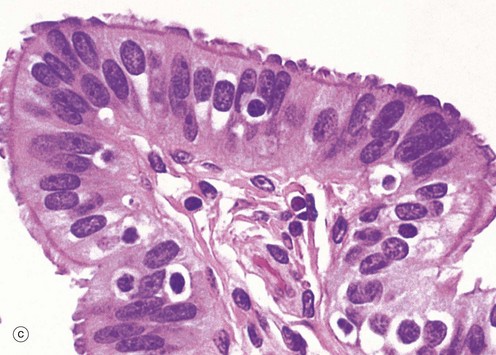
At the time of ovulation, the infundibulum moves so as to overlie the site of rupture of the Graafian follicle. Finger-like projections called fimbriae extending from the end of the tube envelop the ovulation site and direct the ovum into the tube. Movement of the ovum along the tube is mediated by gentle peristaltic action of the longitudinal and circular smooth muscle layers of the oviduct wall. This is aided by a current of fluid, propelled by the action of the ciliated epithelium lining the tube. The mucosal lining of the Fallopian tube is thrown into a labyrinth of branching longitudinal folds, a feature that is most prominent in the ampulla (a), which is the usual site of fertilisation. Note also in this micrograph, the muscular wall M and the vascular supporting tissue of the serosa S, which is continuous with the broad ligament BL. The serosal layer and broad ligament have a surface lining of mesothelium. The muscular wall has two layers, an inner circular and an outer longitudinal, not discernible at this magnification.
Micrograph (b) focuses on one of the mucosal folds of the ampulla. These have a branching core of vascular supporting tissue ST and are invested by a single layer of tall columnar epithelial cells E.
Micrograph (c) shows the tip of a mucosal fold at high magnification. The columnar cells of the epithelium are of three types: ciliated, non-ciliated secretory and intercalated cells. The non-ciliated cells produce a secretion that is propelled towards the uterus by the wave-like beating of the cilia of the ciliated cells, carrying with it the ovum. This secretion probably also has a role in the nutrition and protection of the ovum. The intercalated cells may be a morphological variant of the secretory cells. The ratio of ciliated to non-ciliated cells and the height of the cells undergo cyclical variations under the influence of ovarian hormones. The ciliated cells are generally shorter than the secretory cells, making the epithelial surface somewhat irregular in outline. Scattered intraepithelial lymphocytes are also present.
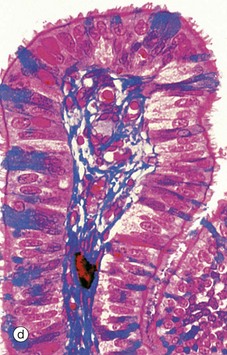
Micrograph (d) employs a method that stains the secretory cells blue. Note that the collagen of the supporting tissue core of the mucosal fold is also stained blue.![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Basicmedical Key
Fastest Basicmedical Insight Engine