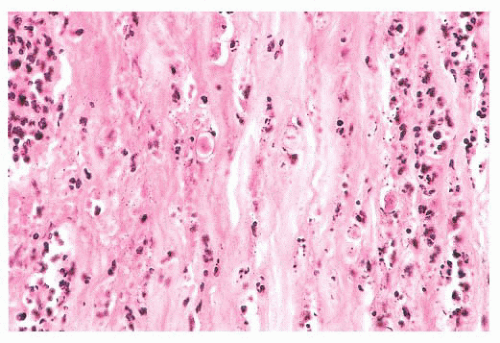
significant tissue response. Intraocular foreign bodies containing iron are particularly toxic to the retina, and they may cause a diffuse deposition of iron throughout the eye (13). Copper-rich foreign bodies incite a significant intraocular acute inflammatory reaction (14). Other metals such as lead, zinc, nickel, aluminum, and mercury may also evoke intraocular inflammation.
TABLE 24.1 Benign Intraocular Tumors | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||
TABLE 24.2 Causes of Intraocular Granulomatous Inflammation | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||
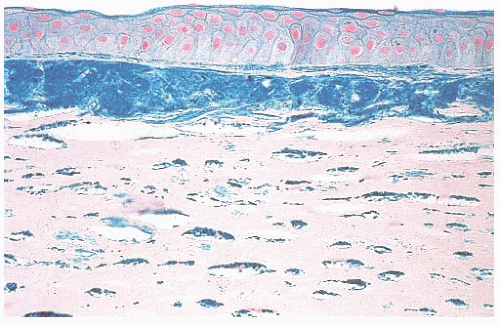
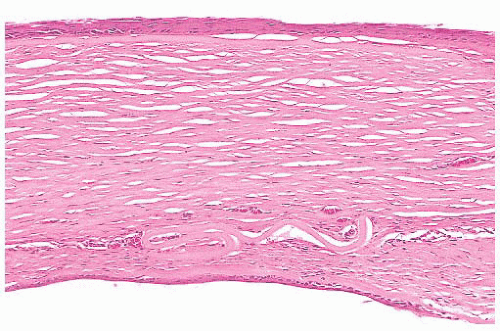
optic nerve is characterized by an accumulation of hyaluronic acid that stains positively with the Hale colloidal iron technique or the Alcian blue stain (Schnabel cavernous atrophy). An examination of a glaucomatous eye may also disclose the cause of congenital or secondary glaucoma. For example, fibrocollagenous adhesions may be present between the posterior surface of the peripheral cornea and the iris (peripheral anterior synechiae), and this may have obstructed the aqueous humor outflow.
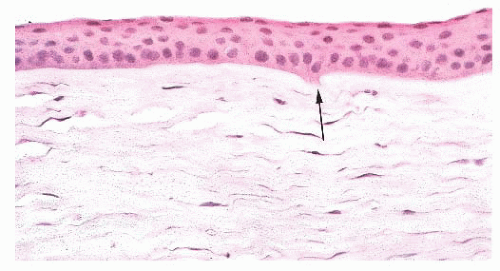
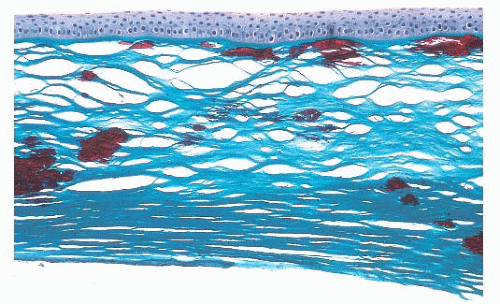
associated with inflammation. With aging, corneal endothelial cells diminish in number, and Descemet membrane thickens. A diffuse, irregular thickening of Descemet membrane accompanies some long-standing degenerative changes of the endothelial layer. Fibrous retrocorneal membranes between the Descemet membrane and the corneal endothelium may follow grafts or various inflammatory processes of the cornea (46).
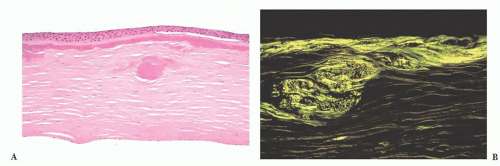
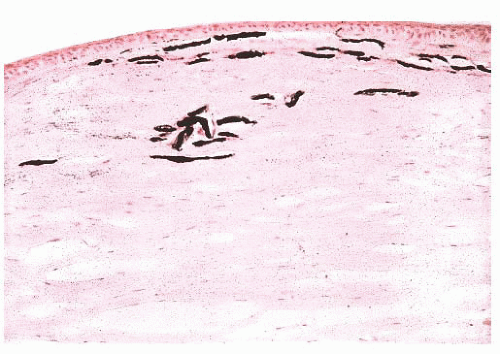 FIGURE 24.4 Calcific band keratopathy. Linear deposits of calcium phosphate are present within the superficial corneal stroma (von Kossa stain). |
to specific chromosomes, and the genes have been identified in many of them. Different genetic mutations have been related with CDs development. Most frequently involved genes are TGFBI, CHST6, KRT3, KRT12, PIP 5k3, SLC 4AIII, TATICSt2, and UBIADI.
TABLE 24.3 Significant Features and Predominant Corneal Layer Affected in Some Corneal Dystrophies | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
TABLE 24.4 Stains Used and Patterns for Histopathologic Diagnosis of the Stromal Corneal Dystrophies | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||
causative microorganism is often difficult to detect in tissue sections without the aid of special stains. Colonies of some bacteria, such as Streptococcus viridans, may produce crystalline-like stromal opacities in the absence of an inflammatory cell infiltrate (“infectious pseudocrystalline keratopathy”) (81).
TABLE 24.5 Some Infectious Organisms in Keratitis | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||
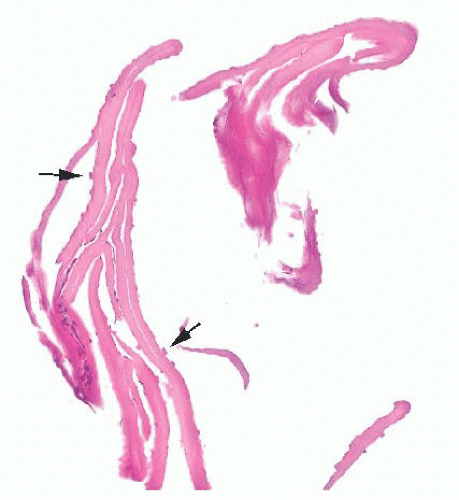
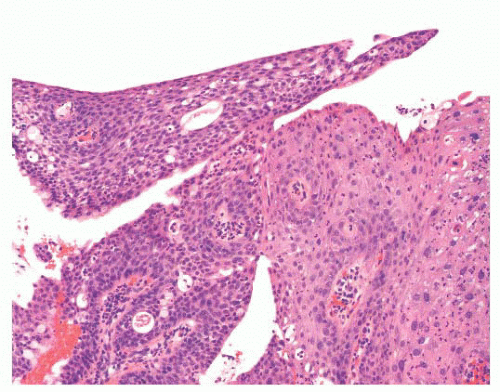
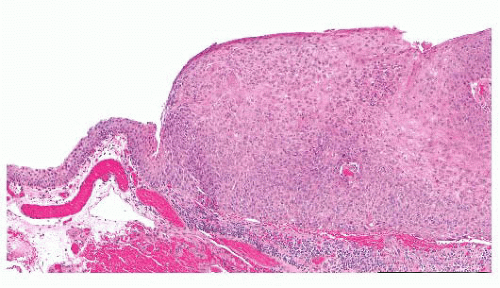
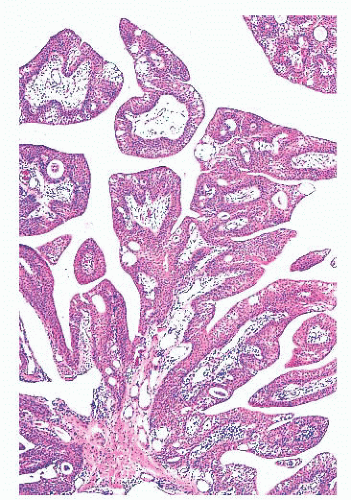 FIGURE 24.14 Conjunctival papillomas. Lobules of squamous epithelium surrounding a fibrovascular core characterize these papillomas histologically. |
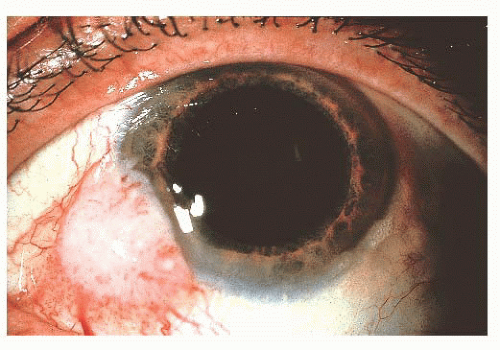 FIGURE 24.17 Squamous cell carcinoma of the conjunctiva. These most often arise at the corneoscleral limbus. |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree